130 JOURNAL OF COSMETIC SCIENCE was performed only once without overlapping a new light beam in areas that were already treated. All applications were performed under cooling analgesia with the Siberian-Fit® device, serial number 06375-14, Anvisa Registration 80058589003, which offers comfort to the patient during the procedure. As a result, the treatment was without pain. After the application, there was a recovery period consequent to the inflammatory process that IPL causes in all treated patients. We evaluated the anti-inflammatory effects of the cream containing 0.5% P. angulata L. extract in comparison to 1% hydrocortisone and vehicle alone. IMPLEMENTATION The participants (n = 60) were divided into two groups, G1 (n = 30) and G2 (n = 30). After the IPL application, each group received different treatments on the left and right hands as follows: • G1-PA: IPL was applied to the left hand and treated with a cream identified with a red label, which contained 0.5% P. angulata L. extract (n = 30 left hands). • G1-H: IPL was applied to the right hand and treated with a cream identified with a yellow label, which contained 1% hydrocortisone (n = 30 right hands). • G2-PA: IPL was applied to the left hand and treated with a cream identified with a green label, which contained 0.5% P. angulata L. extract (n = 30 left hands). • G2-V: IPL was applied to the right hand and treated with a cream identified with a blue label, which contained vehicle only (n = 30 right hands). The creams were provided to the participants. The participants were instructed to use the cream labeled with the color corresponding to the wristband that they received (Figure 3). The participants were instructed about the amount of cream to be applied (Figure 3) and to wash their hands only twice a day with a nonastringent lotion available in the market (Cetaphil®). Participants used the cream twice a day, right after washing their hands, for 48 h after the IPL application. This time duration was determined because the inflammation caused by IPL application improves spontaneously after 48 h. All participants were instructed to rest, avoid any kind of trauma and proximity to the sun or any other heat-emitting source, and not to handle chemical products of any nature. After 48 h, the participants returned to the doctor’s office, and the bottles were weighed again. The hands were photographed again, following the same norms of the initial procedure, and the patients were examined by the physician using an evaluation questionnaire based on subjective and objective criteria (Chart 1). The analysis of objective criteria was performed by the physician during which the extent of perilesional erythema (in mm) around the melanosis of each hand (which had been previously marked and photographed) was measured (Figures 1 and 2). The measurements were made with a portable dermatoscope (Dermlite 3Gen®) with diode illumination that allowed 10 times magnification (Figure 4). Temperature was measured with a Microlife digital infrared thermometer (serial number 521500398). To avoid measuring different points on each hand, the area of melanosis that was marked and had a similar location on both hands was chosen. The presence of edema
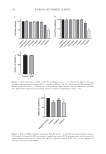
131 PHYSALIS ANGULATE CREAM FOR SOLAR MELANOSIS was verified and classified as absent, present and discrete, present and moderate, or present and intense. Blisters were also observed and classified as present or absent. The subjective criteria were reported by the participants and included pain (classified as absent, present and mild, present and moderate, and present and intense), pruritus (also classified as absent, present and mild, present and moderate, and present and intense), and asking the participant which hand felt better (right, left, or no difference between them). See Table I. Results were analyzed using the Bioestat 5.0 program. The differences between the treatments for the parameters analyzed were compared using simple analysis of variance (ANOVA). When relevant, the means were compared by the t-test. The results, expressed as mean ± standard error of the mean (Mean ± SEM), were considered statistically significant when p ≤ 0.05. RESULTS G1 (Figure 5) compared 0.5% P. angulata L. extract with 1% hydrocortisone. In G1-PA, five participants (16.6%) reported mild pain and three participants (10%) had mild pruritus. Pain and pruritus were not reported on the hands of G1-H participants. Skin temperature was higher in G1-PA in 20 participants (66.6%), and in 5 participants, the elevation was 0.5°C but 1°C and was not significant (p = 0.261). In G1-H, six participants (20%) had higher temperature and four (13.3%) participants had no changes. Figure 3. G2 participant with an illustration of the amount of cream to be applied marked according to the colors of bracelets and bottles as well as the soap offered to wash their hands.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)