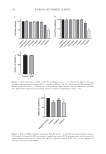
146 JOURNAL OF COSMETIC SCIENCE (0.3 g/L), which was used as the interfering substance for the test conditions. After 60 seconds contact time, a neutraliser was immediately added to stop the effects of the disinfectant, and a sample of the corresponding mixture was poured into a bacterial medium plate and incubated. After incubation, the number of surviving bacteria was counted and compared against the original culture number. A 5 log (base 10) reduction within all 4 species met the formally established standard, although reductions below this level and/or in less than 4 species may (also) be indicative of antimicrobial activity. RESULTS FORMULATION DEVELOPMENT When the lotion prototype version 1.0—containing 20% w/w IR3535 and according to the generic ingredients list of Table I—was subjected to stability testing at RT and accelerated temperatures (e.g., 40°C and/or 50°C), it manifested rapid reductions from the initial pH over the course of weeks to months. The reductions in pH by 1.0 units occurred within 5.1 weeks at 40°C and 16.4 weeks at RT. A variance of pH of 1.0 units or greater within this predetermined time (i.e., 12 months at RT) was deemed as an undesirable property it may be a key factor used as a determinant of the imputed or formal shelf life. The pH drift underscores that some component(s) of the prototype version 1.0 were undergoing chemical hydrolysis or degradation, as evidenced by increased concentration of hydronium ions (H3O+) or protons, detected by a pH meter. Lower pH and/or the presence of degradants within the formulations might be disadvantageous to human or animal skin. Note that none of the initial formulation variants, including version 1.0, contained trisodium citrate, although some variants contained citric acid as an optional acidifier/antioxidant. According to the manufacturer of IR3535 (Merck KGaA), the ideal pH for IR3535 is between 6.0 and 6.5. A challenge of using IR3535 as the selected active ingredient in these encapsulated repellent formulations is that in order to adequately solubilize, suspend, or emulsify the IR3535 and the other essential excipients, a pH lower than its inherent ideal pH of IR3535 can be desirable. For human skin, a pH of approximately 5.0–6.5 is appropriate, and approximately 5.5 is often preferable or optimal in topical consumer products. These two parameters are in apparent opposition logic suggests that a low pH might be desirable in the process of producing an IR3535-containing encapsulated formulation, but a higher pH (approximately 5.0–6.5) is desirable for application of the formulation to human skin to avoid unnecessary skin irritation. It follows that the manufacturing process steps (e.g., sequence of addition of the ingredients, temperatures, and mixing intensity) in preparing the formulations may also impact ingredient solubilization, suspension, emulsification, and encapsulation in a pH-dependent manner. Thus, empirical formulation development and analyses via a reiterative process can improve from an initial prototype formulation toward preferred formulations. In multiple efforts to ameliorate the reductions in pH and concentration of the active ingredient, attempts were made to stabilize the formulation(s) with: (a) addition of NaOH to increase the initial pH (b) addition of NaOH to restore (increase) the pH after the formulation had manifested some degree of pH loss and (c) addition of an antioxidant, with the intent of retarding the chemical instability. None of these three empirical approaches
147 MICROENCAPSULATED INSECT REPELLENT adequately addressed the chemical instability issue(s). The instability persisted even when NaOH or an antioxidant was included. It is likely obvious to an experienced formulation scientist that the back neutralization of a citric acid–containing solution (or emulsion) with sodium hydroxide is inherently equivalent to the use of a sodium citrate (or hydrates thereof) buffer solution, differing only in order of addition of the component parts. Nevertheless, this approach did not resolve the issue of pH getting lower over time. The concentration of IR3535 within prototype version 1.0 was assayed at various time points using gas chromatography. The analysis revealed that the degradation of IR3535 at 24 months at 20°C exceeded the WHO and EPA guidelines’ allowed tolerances of 5% to 6% for repellent products, with a declared nominal content of active ingredient of 10% to 25% w/w or w/v and stored at 20°C ± 2. These findings (as previously mentioned) further underscored the problems and confounding variables to be addressed. In summary, the problematic issues faced during formulation development included: (a) most notably rapid pH loss (b) loss of IR3535 (c) a preference for a lower pH for solubility, suspension, emulsification, and encapsulation during formulation development (d) preference for human skin of a pH of approximately 5.0–6.5, and more preferably 5.5 and (e) attempts to maintain appropriate pH conditions by the addition of NaOH or an antioxidant not being sufficient. Thus, multiple attributes of the excipient components, concentrations, and sequence of addition (order) were evaluated to optimize the desired formulation. To prevent the rapid pH reduction manifested by prototype version 1.0 during stability testing (see following section), a formulation version 2.0 was prepared that was essentially prototype version 1.0 but that included trisodium citrate dihydrate (2.024% w/w) plus citric acid (0.095% w/w). This buffered formulation contained a total of 30.63% active ingredient plus excipients, with an initial pH of 6.2. Formulations with two chemical versions of sodium citrate were studied in the dihydrate form and the monohydrate form. To maintain equimolar citrate ion concentrations, less of the monohydrate was required relative to the dihydrate. The dihydrate form was routinely used in commercial consumer products and foods, was less expensive, and may be preferable for the formulation(s). Formulation variants were also prepared with a concentration range of sodium citrate– citric acid to identify preferred amounts of the buffer. Concentrations ranged from 0.2% to 5.0%. In general, the optimal concentration for chemical stability and physical aspects was approximately 2%. Formulation version 2.55 was identical to version 2.0, except it contained 1.80% trisodium citrate dihydrate: 0.31% citric acid, at initial pH 5.5, and 69.38% water. TESTING OF STABILITY AND PHYSICAL ASPECTS A major goal during formulation development was to limit the reduction in pH to no more than 1.0 units within 12 months (and preferably longer) at RT. This desirable increased pH stability was also expected to stabilize the concentration of IR3535, so that it did not exceed 5% to 6% loss over the imputed or formal shelf life. The slopes of the linear regressions (with r2 values 0.9) were extrapolated into imputed (estimated) weeks to reach a pH loss of 1.0 units—thus, from an initial pH of 6.2 downward to 5.2 for version 2.0, or from an initial pH of 5.5 downward to 4.5 for version 2.55. Table II reveals that linear regression analyses of weekly measurements of the unbuffered repellent lotion prototype version 1.0 manifested rapid reductions in pH by 1.0 units within
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)