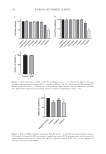
162 JOURNAL OF COSMETIC SCIENCE It is well-known that kojic acid, arbutin, and hydroquinone significantly inhibit melanin biosynthesis (22). Many polyphenol derivatives show excellent antimelanogenesis potency through interactions with tyrosinase via noncovalent interactions (23), as well as due to their structural specificity. Notoginseng, the root of Sanchi, was first used by minority ethnic groups to promote blood circulation and to relieve pain in southwestern China (24). However, there are few reports about Sanchi flower extracts. Saponin, the main component of Sanchi flower extract, contains many hydroxyl groups in its structure, which may interact with tyrosinase through various noncovalent bonds, therefore inhibiting the production of melanin. In this study, SFE showed antimelanogenic potency by suppressing intracellular tyrosinase activity, which was similar to that of arbutin (1 mg/mL) in B16-F0 cells. The further mechanism of action of the extract deserves more studies. The ROS caused by UV exposure is one of the factors that can promote melanin generation, melanocyte proliferation, and DNA damage (25). To ensure ROS at normal levels (26), intracellular antioxidant capacity was regulated in different types of skin cells through natural antioxidant systems, including GSH-Px, catalase, and superoxide dismutase. UV exposure reduced the activity of antioxidant enzymes in HaCaT cells, while treatment with SFE could increase the GSH-Px and T-AOC capacity, which is consistent with vitamin C. Therefore, natural antioxidant and antityrosinase activity helped reduce skin damage. Current in vitro pathophysiological studies are mainly performed in cell models, in which cells are cultivated as monolayers on a solid surface. Cell culture systems play an important role in further understanding cell morphology, molecular signaling, and drug discovery (27), but the results and conclusions from these 2D cell culture systems are not entirely suitable for in vivo physiological systems. In addition, 2D monolayers particularly lack environmental factors that are relevant to the 3D in vivo environment. The limitations of 2D cell culture models as unreliable predictors of drug efficacy and toxicity in vivo are supported by relatively high drug failure rates in preclinical trials for diseases (28). Therefore, physiological 3D skin models are required to provide a better platform for evaluating biological efficacy for cosmetics and drugs (29). MelaKutis has a highly consistent histological structure and melanin response function with human skin, which can accurately reflect the whitening effect of the extract on the human body. In this study, the 3D skin model had obvious hyperpigmentation after UV irradiation. However, the 3D models after treatment with SFE significantly brightened, and the melanin content was obviously reduced. These results indicated that SFE might be able to achieve the same whitening effect on the human body. The authors discovered that SFE could significantly inhibited cellular melanin production and tyrosinase activity in a B16-F0 cell. Its antimelanogenesis effects in the 3D skin model were consistent with those in a 2D cell system. The extract showed good antioxidant activity targeting GSH-Px activity and T-AOC in HaCaT cells exposed to UV radiation. This might reduce the oxidation reactions associated with melanin production, thus preventing melanin synthesis. In addition, many studies found that kojic acid was mainly complexed with Cu2+ on the active center of tyrosinase (30,31) through the 5-position hydroxyl group and the 4-position ketone group, thus inhibiting enzyme activity. Meanwhile, there were many hydroxyl groups in the active ingredient of SFE, which showed the potential to complex with copper ions in tyrosinase. Therefore, SFE might have a similar whitening mechanism compared to kojic acid, which is worthy of further exploration in future research. In general, SFE might be a potential antioxidant and antimelanogenesis candidate.
163 ANTIPIGMENTATION AND ANTIOXIDANT ACTIVITY CONCLUSION It is well-known that UV exposure can trigger oxidative stress, and the superfluous ROS accumulation promotes melanin synthesis and activates tyrosinase in melanocytes. In this study, SFE effectively alleviated UVB-induced oxidative stress by increasing GSH-Px and T-AOC activities in epidermal keratinocytes. It also exhibited potent inhibitory activity on melanin production caused by UVB exposure in a 3D skin model, which suggested that it might have a similar whitening effect on human skin. Above all, it is suggested that SFE will expand its applications in cosmetic manufacture. ACKNOWLEDGMENTS This work was supported by the Yunnan Science and technology project (2018ZF005). REFERENCES (1) H. Liu, X. Lu, Y. Hu, and X. Fan, Chemical constituents of Panax ginseng and Panax notoginseng explain why they differ in therapeutic efficacy, Pharmacol. Res., 161, 105263 (2020). (2) B.R. Yang, S.C. Yuen, G.Y. Fan, W.H. Cong, S.W. Leung, and S.M. Lee, Identification of certain Panax species to be potential substitutes for Panax notoginseng in hemostatic treatments, Pharmacol. Res., 134, 1–15 (2018). (3) L. Peiran, L. Ying, Z. Mingzhuo, Y. Ye, and C. Xiuming, The development of a Panax notoginseng medicinal liquor processing technology using the response surface method and a study of its antioxidant activity and its effects on mouse melanoma B16 cells, Food Func.., 8(11), 4251–4264 (2017). (4) H. Dumbuya, S.Y. Hafez, and E. Oancea, Cross talk between calcium and ROS regulate the UVA- induced melanin response in human melanocytes, FASEB J., 34(9), 11605–11623 (2020). (5) J.O. Lee, E. Kim, J.H. Kim, Y.H. Hong, H.G. Kim, D. Jeong, J. Kim, S.H. Kim, C. Park, D.B. Seo, Y.J. Son, S.Y. Han, and J.Y. Cho, Antimelanogenesis and skin-protective activities of Panax ginseng calyx ethanol extract, J. Ginseng. Res., 42(3), 389–399 (2018). (6) Y.C. Hseu, Y. Vudhya Gowrisankar, L.W. Wang, Y.Z. Zhang, X.Z. Chen, P.J. Huang, H.R. Yen, and H.L. Yang, The in vitro and in vivo depigmenting activity of pterostilbene through induction of autophagy in melanocytes and inhibition of UVA-irradiated α-MSH in keratinocytes via Nrf2-mediated antioxidant pathways, Redox Biol., 44, 102007 (2021). (7) S.H. Hashemi-Shahri, A. Golshan, S.A. Mohajeri, J. Baharara, E. Amini, F. Salek, A. Sahebkar, and Z. Tayarani-Najaran, ROS-scavenging and anti-tyrosinase properties of crocetin on B16F10 murine melanoma cells, Anticancer Agents Med. Chem., 18(7), 1064–1069 (2018). (8) J. Kim, Y.H. Kim, S. Bang, H. Yoo, I. Kim, S.E. Chang, and Y. Song, L-765,314 suppresses melanin synthesis by regulating tyrosinase activity, Molecules, 24(4), 773 (2019). (9) S.Y. Shim, Y.E. Lee, and M. Lee, Antioxidant compounds, kirenol and methyl ent-16α, 17-dihydroxy- kauran-19-oate bioactivity-guided isolated from Siegesbeckia glabrescens attenuates MITF-mediated melanogenesis via inhibition of intracellular ros production, Molecules, 26(7), 1940 (2021). (10) A.R. Im, S.H. Yeon, J.S. Lee, K.A. Um, Y.J. Ahn, and S. Chae, Protective effect of fermented Cyclopia intermedia against UVB-induced damage in HaCaT human keratinocytes, BMC Complement. Altern. Med., 16, 261 (2016). (11) A.Y. Lee, Skin pigmentation abnormalities and their possible relationship with skin aging, Int. J. Mol. Sci., 22(7), 3727 (2021). (12) Y. Zi, B. Zhang, B. Jiang, X. Yang, Z. Liang, W. Liu, C. He, and L. Liu, Antioxidant action and protective and reparative effects of lentinan on oxidative damage in HaCaT cells, J. Cosmet. Dermatol., 17(6), 1108–1114 (2018). (13) T. Fu, B. Chai, Y. Shi, Y. Dang, and X. Ye, Fargesin inhibits melanin synthesis in murine malignant and immortalized melanocytes by regulating PKA/CREB and P38/MAPK signaling pathways, J. Dermatol. Sci., 94(1), 213–219 (2019).
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)