333 Application of Reverse Micelles in Cosmetic Formulations Several factors must be considered to obtain the desired micellar systems. Molecular structures of surfactant molecules, mobility of surfactant in the solvent, and reversibility or adjustability during the self-assembly process must be considered. Micellization conditions such as solution pH, ionic strength, and temperature may also cause different aggregates to form. Morphology of surfactant aggregates can be predicted by using a packing parameter as shown in following equation: p V a l hc c =0 Where p =packing parameter, V hc =the volume of hydrophobic chain, a 0 =mean cross- sectional area of the head group in the aggregate, and l c =length of the fully extended chain. The various morphologies that formed at different values of the packing parameter are given in Table I. The equation shows that morphology of micelles depends mainly on the hydrocarbon chain length and the dimension of the head group. Reverse micelles are formed at a p value larger than 1. The shape of reverse micelles can be spherical or cylindrical depending on the compositions of the micellar systems. Understanding the micellization behavior of a surfactant system is helpful in determining its potential applications. Some commonly used methods to investigate the micellization process are transmission electron microscopy, dynamic light scattering, small angle neutron scattering, and fluorescence quenching. Simulation techniques like dissipative particle dynamics methods and molecular dynamics are also used to study the micellization process. Models can be developed based on free energy change during micelle formation to predict the properties of micelles (19). These experimental and simulation techniques can provide data to deduce the interactions between surfactant molecules, surfactant-solute interactions, and the solubilization site of the solute. Rheological changes such as micellar shape and viscosity of the system at different temperatures, ionic strength, pH, and presence of additives or impurities can be studied through those techniques. In mixed surfactant systems, the balance between molecular interactions, such as electrostatic interactions and hydrophobic interactions, also affects the micellization behavior. It is important to design cosmetic formulations with most suitable surfactants, optimized compositions, desired properties, and lower production costs. NORMAL MICELLE Normal micelles form when surfactant concentration in an aqueous solution reaches its CMC. Hydrophobic interactions are the main driving force of micellization (20). The TABLE I Morphology of Surfactant Aggregates at Different Packing Parameters Packing parameter, p Morphology of aggregates 1/3 Spherical micelle 1/3–1/2 Cylindrical micelle 1/2–1 Vesicle Around 1 Planar bilayer 1 Reverse micelle
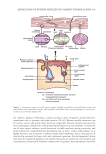
334 JOURNAL OF COSMETIC SCIENCE structure of normal micelles resembles core-shell structures where the core is composed of oil phase or hydrophobic solvent. Surfactant molecules align themselves so that their hydrophobic tails point toward the micellar core and their hydrophilic head groups at the micellar surface. The shape of micelle can be spherical or cylindrical depending on their p. The hydrophobic core can encapsulate hydrophobic molecules, thus significantly increasing their solubility in aqueous solutions. More hydrophobic molecules are generally located deeper inside the micellar core during encapsulation. Fewer hydrophobic molecules may solubilize near the oil-surfactant interface or in the palisade layer of the micelle. The structure of a spherical micelle encapsulating hydrophobic molecules is shown in Figure 1. The micellization process largely depends on the concentration of surfactant in the system (8). The number of micelles formed generally increases with surfactant concentration. High surfactant concentration may lead to micellar growth. The micelles formed are generally classified as spherical with their average diameter about double the diameter of the amphiphile surfactant molecule (6). Formation of three-dimensional, cubic micellar structures was reported when a high surfactant concentration is used (7). Temperature is another important factor affecting micellization and the structural morphology the micelles formed (16). This is related to the variation in the strength of the interactions between the surfactant molecules at different temperatures. Other factors such as surrounding pH, salt concentration, and the presence of additives may also influence the properties of the micellar system. Micelles can act as nano-sized carriers to transport active ingredients across skin. Hydrophobic active compounds can be delivered to a targeted spot by encapsulation within the micelles. The controlled release of active ingredients from the micelles can be achieved through proper formulation design (8). This allows delivery of active ingredients that are normally unable to penetrate the skin. In cosmetic applications, the size of the micelle is an important factor because only micelles with a smaller diameter than the pores of the Stratum corneum (SC) can penetrate the skin barrier, while larger micelles are obstructed from penetrating the skin (21). However, micelles do not necessarily need to penetrate the skin to deliver the active compounds. Micelles may disintegrate on the skin surface and in the hair follicles to release their contents into deeper skin layers. In cosmetic formulations, essential oils and hydrophobic active ingredients are added to skin care products, such as toner, through solubilization in micelles (9). Functions of the active ingredients in micelles are preserved and their stability can be improved, allowing the products to be stored for longer duration. Figure 1. Structure of a spherical micelle encapsulating hydrophobic molecules (7).
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)