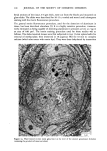
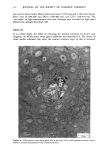
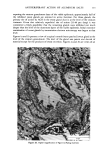
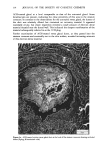
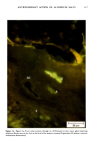
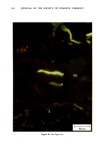
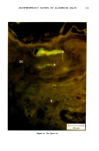
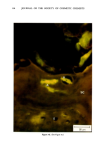
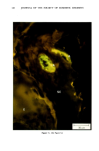
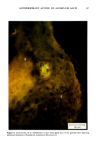
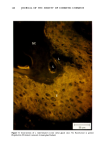
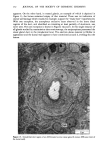
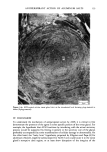
j. Soc. Cosmet. Chem., 32, 107-136 (May/June 1981) The mechanism of antiperspirant action by aluminum salts. II. Histological observations of human eccrine sweat glands inhibited by aluminum chlorohydrate RICHARD P. QUATRALE, PH.D., DON W. COBLE, KARLA L. STONER, and CARL B. FELGER, PH.D., Gillette Research Institute, 1413 Research Blvd., Rockville, MD 20850 Received October 12, 1979. Presented at the SCC Annual Scientific Meeting, December 11 & 12, 1980, New York, N.Y. Synopsis Human forearm biopsy tissue containing eccrine sweat glands which had been inhibited from firing by the occlusive application of aluminum chIorohydrate (ACH) were examined histologically. The objective of the study was to determine the presence and site of action of the antiperspirant within the sweat gland as a prerequisite to understanding its mechanism of action. Transmission electron microscopy studies of ACH-treated eccrine sweat glands revealed that just as the duct leaves the stratum granulosum, and then as it passes through the stratum corneum to the skin surface, its lumen is completely filled with an electron-dense amorphous material. Similar material was frequently observed in the duct at the upper layers of the viable epidermis, and in a few instances at the level of the basal layer of the epidermis. However, it was found as deep as the upper dermal layer only once. This emphractic material is believed to consist of ACH or one of its reaction products and thought to be responsible for sweat gland inhibition. Alternatively, untreated sweat glands were always patent and devoid of comparable material. Fluorescence microscopy studies of morin dye-stained biopsy tissue revealed substantial fluorescence, indicating the presence of aluminum within the sweat gland duct at the level of the stratum corneum. Occasionally, fluorescence was also observed within the duct at the level of the stratum granulosum. Anatomically, the aluminum present within the sweat gland, as demonstrated with morin dye, was at the same site as the electron dense amorphous material demonstrated with TEM studies. Fluorescence was never observed in untreated sweat glands. I. INTRODUCTION The use of aluminum salts for reducing or eliminating eccrine sweating has been recognized by the scientific community for many years. Despite a long and extensive history of use, the anathema of their incompletely understood mechanism of action still largely persists. Recently, various theories put forth by investigators have been summarized (1,2). These proposed mechanisms, nevertheless, are far from having irrefutable supporting evidence, as testified by the very fact that no singular mechanism enjoys uncontested acceptance. 107
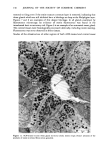
108 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS Two aluminum-based salts, aluminum chlorohydrate (ACH) and the aluminum zirconium chlorohydrate glycine complexes, are currently widely marketed as antiper- spirants. For the more extensively used form, ACH, there is little information describing its mechanism of action. To understand the mode of action by a chemical on a biological system, or even to offer a plausible hypothesis regarding its action, the site of action should first be known. Recently, it was shown that for at least half the eccrine sweat glands in a population of glands occlusively treated with 20% ACH, the site of action within the sweat gland duct is not deeper then the stratum corneum layer of skin. Removal of the stratum corneum layer, by stripping with Scotch tape, results in the restoration of sweating in 5096 of those glands previously shown to be inhibited (3). Relier and Leudders have provided histological evidence, at least for AICI 3 and an aluminum-zirconium solution, that sites of action are as deep as the intradermal duct and as superficial as the stratum corneum level respectively (2). They proposed that the mechanism of action of these two salts, as well as that of ACH and other metal salt antiperspirants, is via the formation of an obstructive hydroxide gel within the sweat gland duct. However, no supporting histological evidence indicating the precise location of ACH was provided. Using both transmission electron microscopy (TEM) and fluorescence microscopy in combination with morin dye staining techniques, we have now been able to demonstrate that human sweat glands, which have been inhibited by treatment with ACH, contain within the uppermost levels of their lumens, an electron-dense mass consisting at least partially of aluminum. II. MATERIALS AND METHODS A. TREATMENT PROTOCOL AND BIOPSY OF TISSUE In the early afternoon of Day 1, one site on the volar surface of each forearm, 2-3 cm proximal to the wrist where the most dense sweat gland population is known to be present, was selected on each of five male subjects. Each site was shaved free of hair, if necessary, and cleaned with 95% aqueous ethanol. A 2.5 x 2.5-cm square was marked on the skin site with indelible ink. The subjects next entered an environmental chamber set at 100øF and 30% R.H. for thermal stress. A mixture of starch, castor oil and 2% iodine in ethanol, a modification of the original method of Wada and Takagaki (4), was applied to the forearm sites to visualize the sweat droplets. Following development of a suitable sweating pattern, several specific fields were selected within the site, each having an area of 25-50 mm 2. Their exact location was recorded using an overlaying grid. Only fields with a high density of actively firing sweat glands were chosen. The subjects then returned to ambient temperature and the starch mixture was removed. One site for each subject, designated "experimental," was then occlusively patched with 20% aqueous ACH. The other site was occlusively patched with distilled water. The subjects were dismissed with instructions to remove the patches from the sites approximately 16-18 h later. The subjects returned to the laboratory in the late morning of Day 2. After ascertaining that the patches had been removed from the sites at least three hours earlier, so that no sweat gland inhibition due to skin hydration would be manifest, they
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)