ANTIPERSPIRANT ACTION OF ALUMINUM SALTS 109 were thermally stressed again using the starch-iodine mixture to visualize the sweating pattern. When the sweating pattern had developed, each ACH-treated site was examined closely for areas of complete sweat gland inhibition. Again using the overlaying grid, one of the fields identified the previous day was selected for subsequent biopsy. The choice of field was based not only on that field having had an ample number of actively firing glands prior to treatment, but more importantly, ascertaining that all glands in that field had been inhibited from firing by the ACH treatment. With this safeguard, the eventual histological examination of only those glands known to have been inhibited could be performed with certainty. The control sites on each subject were similarly examined, and a field in each was selected for biopsy. In this instance, however, the presence of actively firing glands both before and after placebo treatment was the basis for choice. The starch-iodine mixture was then removed and the subjects left the environmental chamber. Following appropriate presurgical preparation of the skin sites, the precise field for biopsy was identified, once again with the aid of the overlaying grid. A full thickness skin biopsy was taken from each of the sites. The tissue samples were subdivided into 1 x 2 mm blocks. One portion of each biopsy was immediately fixed at room temperature by immersion into 0.1 M Sorensen's phosphate-buffered glutaraldehyde, pH 7.3, for not less than four hours. These samples were subsequently used for TEM studies. The remaining tissue was placed on ice-cold physiological saline-saturated gauze pads in petri dishes until further preparatory steps could be taken for the subsequent examination by fluorescence microscopy. B. PREPARATION OF BIOPSY TISSUE FOR MICROSCOPY 1. Preparation for Transmission Electron Microscopy The tissues to be studied by TEM were removed from the glutaradehyde fixative, post-fixed in 2% phosphate-buffered OSO4, dehydrated in a graded series of aqueous alcohol, cleared in propylene oxide, and embedded in Epon 812 ©. Prior to embedment, the tissues were oriented such that sections would be cut parallel to the skin surface beginning at the secretory coil region of the sweat gland. Serial sections, 600 • thick, were cut with a diamond knife in a Porter Blum MT-2 ultramicrotome, placed on a carbon-backed nitrocellulose-covered single slot copper grid, and stained with both uranyl acetate and lead citrate for image enhancement. Electron microscopy was performed on a JEOL 100B instrument at 80 kV. 2. Preparation for Fluorescence Microscopy The tissues to be subsequently studied by fluorescence microscopy were subdivided into 3 x 3-mm blocks, quickly frozen by direct immersion into liquid N2-cooled 2-methyl butane (isopentane) and freeze-dried. The tissues were then soaked in methacrylate monomer (at a 20:80 ratio of methyl:butyl ester) overnight at 4øC. The samples were brought to ambient temperature and embedded by immersion in BEEM capsules filled with fresh monomer containing 1.5% benzoyl peroxide catalyst. Polymerization was completed by exposure to 55øC for 48 h.
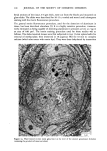
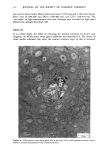
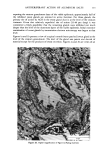
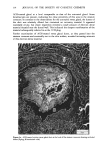
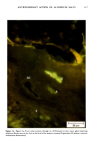
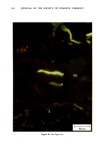
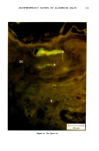
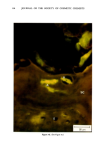
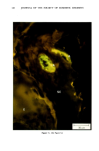
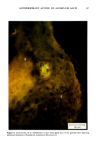
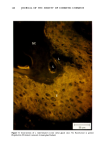
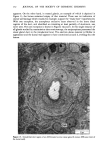
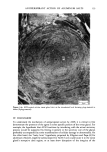
110 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS Serial sections of the tissue, 4-6 gm thick, were cut from the blocks and mounted on glass slides. The slides were heat-fixed for 10-15 s, cooled and stored until subsequent staining with the marin fluorescence procedure. The general marin fluorescence procedure, used for the detection of aluminum in tissue, has been described elsewhere (5). It is a highly sensitive procedure, conserva- tively estimated as being capable of detecting aluminum in amounts as low as 2 pg in an area of 0.03 gm •. The marin staining procedure used for these studies was as follows. The slide-mounted tissues were first subjected to two 15-min xylene baths for removal of methacrylate, then immersed in 1% aqueous HCI for 10 min to complex calcium (which also reacts with marin dye). They were then dehydrated by immersion Figure la. Water-treated eccrine sweat gland duct at the level of the stratum granulosum (L:lumen containing the products of sweat secretion).
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)