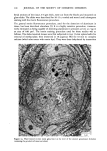
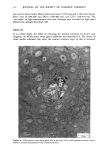
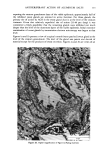
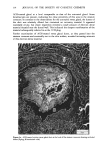
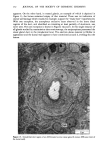
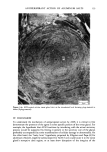
j. Soc. Cosmet. Chem., 32, 153-162 (May/June 1981) A quantitative approach to measuring actinic aging in human skin BARBARA A. GILCHREST, M.D., Department of Dermatology, Harvard Medical School, Beth Israel Hospital, Boston, MA 02215 Received November 12, 1980. Presented at the SCC Annual Scientific Meeting, December 11, 1980, New York, NY. Synopsis Clinically recognized age-associated changes in skin are considerably more prominent in chronically sun-exposed areas and in fair-skinned individuals relatively lacking a MELANIN barrier. PREMATURE AGING due to sun exposure is now widely accepted by dermatologists as well as the lay public, but has proven difficult to quantitate and therefore to study. In the laboratory, it has been possible to demonstrate behavioral differences in cells cultured from both the epidermis and dermis that are almost certainly due to prior sun exposure. Paired skin biopses from the habitually sun-exposed and nonexposed aspects of the arm, obt•iined from nine volunteers aged 28-80 years, were used to establish KERATINOCYTE and FIBROBLAST CULTURES, which were then carried to senescence. In all cases, the number of cumulative population doublings in vitro was greater in cultures derived from nonexposed skin than in those derived from sun-exposed skin, and the discrepancy increased with donor age and severity of clinical aging changes. In the case of the keratinocyte cultures, plating efficiency was higher for cells from sun-exposed skin than for nonexposed controls, perhaps reflecting the recognized carcinogenic potential of actinic radiation. These data demonstrate that existing methodology can quantitatively assess subtle environmental effects on human skin. INTRODUCTION Clinically recognized age-associated changes in skin are wrinkling, loss of elasticity, mottled pigmentation, vascular ectasia, atrophy, and benign proliferative growths such as seborrheic keratoses, acrochordons and cherry angiomata (1). These changes are considerably more prominent in chronically sun-exposed areas and in fair-skinned individuals relatively lacking a melanin barrier. "Premature aging" due to sun exposure is now widely accepted by dermatologists as well as by the lay public, but has proven difficult to quantitate and therefore to study (2,3). Since Hayflick's pioneering work in the 1960s, human diploid fibroblasts have become a widely accepted in vitro model system for gerontologic research by virtue of a finite culture lifespan (4) and certain characteristic, progressive changes in appearance and behavior during cultivation which are presumed analogous to aging at the macro- scopic level (5,6). The model system is further strengthened by the observations that fibroblast lifespan in culture is inversely proportional to the donor's age (4,7) and 153
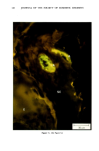
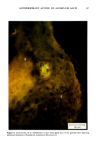
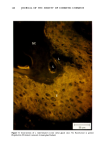
154 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS decreased in individuals with progeria (7-9) Werner's syndrome (7) and diabetes mellitus (7,8,10), all conditions considered by some authorities to manifest premature aging. The Rheinwald-Green technique for cultivation of human keratinocytes utilizing a lethally irradiated fibroblast monolayer has recently for the first time made possible in vitro investigation of a second major cell type in the skin. The following studies were performed in order to determine whether sun-exposed clinically aged skin was indeed "older" than non-exposed skin in the same individual, according to accepted criteria for aging in vitro (7,12). MATERIALS AND METHODS PATIENT MATERIAL Twelve adult male Caucasian volunteers aged 28 to 80 years, known to be in good general health and specifically not to have diabetes, were recruited for this study. All were moderately fair-skinned life-long residents of New England with similar histories of outdoor activities. No subject was using any medication except multivitamins. Biopsies were performed during the winter and early spring months to avoid the possibility of recent sun exposure. Four-millimeter punch biopsies were obtained using local 2% lidocaine anesthesia from normal-appearing skin of the medial (non-exposed) and lateral (sun-exposed) aspects of the upper arm. Informed written consent was obtained from all subjects. CULTURE TECHNIQUES Biopsy specimens were placed immediately in sterile tissue culture medium, coded by a third party, and returned to the investigator, who subsequently did not know which biopsy was chronically sun-exposed and which nonexposed. In the laboratory, subcutaneous fat and deep dermis were dissected away with scissors parallel to the epidermal surface at a depth not exceeding 2.0 mm. The superficial portion, including the epidermis, and the deeper portion, consisting of papillary and superficial reticular dermis, were subsequently handled separately. Keratinocyte Cultures Minor modifications of the procedures described by Rheinwald and Green were used (11). Tissue was minced and placed in 10 ml of .25% trypsin and .01% EDTA in a stirring flask at 37 ø C for 45 min. After allowing the minced biopsy material to settle, the supernatant, containing single cells, was withdrawn and centrifuged for 6 min at 600 rpm, resuspended, counted in a hemocytometer chamber and plated at densities ranging from 104 to 105 cells/60-mm dish in Falcon tissue culture dishes on a monolayer of 3T3 fibroblasts which had been lethally irradiated and plated the preceeding day. In most cases a total of 105 to 106 cells was obtained. All cultures were maintained in Dulbecco's modified Eagle's medium with 20% fetal calf serum, hydrocortisone 0.4 ng/ml, penicillin and streptomycin. Medium was changed five days after innoculation and twice weekly thereafter.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)