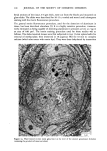
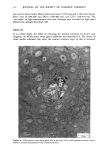
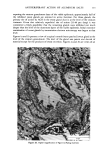
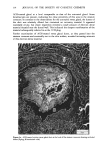
ACTINIC AGING IN SKIN 161 radiant energy which is primarily responsible for sunburn, tanning, vitamin D synthesis, and the skin's other recognized actinic responses (13). Certainly, the severity of actinically induced clinical changes in the sun-exposed sites paralleled the subsequent reduction in lifespan of the cultured cells. The present study allows no insight into the mechanism by which chronic sun exposure in vivo decreases the subsequent in vitro lifespan of cultured cells. Repeated exposures may, however, stimulate mitosis, and thus result in fewer "reserve" cell divisions in sun-exposed vs. nonexposed skin sites to be manifested in culture after biopsy. Alternatively, sun exposure may sublethally injure selected keratinocytes and fibroblasts in such a way that their ability to divide is severely limited, decreasing the average growth potential of the cell population. An unexpected finding was the greatly increased plating efficiency of chronically sun-exposed keratinocytes. These cells were obtained by definition from the lateral, as opposed to the medial, aspect of the arm, but it is difficult to attribute this altered behavior in culture to innate differences between skin specimens from these adjacent sites. Plating efficiency in this system is the number of non-terminally differentiated keratinocytes in the basal layer of the epidermis which form visible colonies in culture, divided by the total number of cells plated. The latter number includes total basal layer keratinocytes (the "true" or desired denominator) plus melanocytes, differentiated keratinocytes in the suprabasalar layers, dermal fibroblasts, endothelial cells, mast cells, and histiocytes. Chronic sun-exposure may influence the thickness of the epidermis, the contour of the dermal epidermal junction and probably to a lesser degree, the number of other cell types in representative cross section of skin. However, neither previous histologic studies (1,14,15) nor examination of representative paired biopsies in this study suggest the up to 30-fold decrease in epidermal and dermal cellular elements in sun-exposed as compared to nonexposed skin that would be necessary to explain this discrepancy anatomically. Rather, it seems that a much greater proportion of those non-terminally differentiated keratinocytes subjected to repeated in vivo solar irradiation are capable of continued cell division in culture, as compared to controls. This increased proliferative capacity of chronically sun-exposed epidermis is interest- ing in two regards. First, most of the clinically apparent actinic changes in skin are proliferative lesions involving one or more cell types: seborrheic keratoses, lentigines, cherry angiomata and telangiectasia (14). Second, repeated exposure to a carcinogen results in a persistently enlarged proliferative compartment in the hamster cheek pouch epidermis in vivo which antedates other evidence of malignant transformation (16), and an increased plating efficiency in vitro for human diploid fibroblasts which are subsequently capable of tumor formation in mice (17). Hence the finding of an increased proliferative pool of keratinocytes in biopsy specimens from chronically sun-exposed skin is consistent with the known carcinogenic effect of actinic irradiation on human skin (18), and measurement of relative plating efficiency for exposed and nonexposed keratinocytes in primary culture may provide a quantitative index of actinic damage or malignant potential. If one accepts the assertion that the in vitro lifespan for fibroblasts (7,20) and perhaps keratinocytes (11) is inversely proportional to the donor's physiologic age, the present data suggest that one effect of chronic sun-exposure on human skin is indeed "premature aging." Further experiments may of course establish that this phenomenon
162 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS represents superposition of environmental damage on intrinsic aging rather than truly accelerated aging, but the fact remains that individually subclinical and temporally remote events (such as sun exposures experienced over many years) can significantly modify the subsequent behavior of cultured cells, and that in the absence of appropriate controls these modifications may be unrecognizable. The current studies strongly suggest that habitual sun-exposure can indeed accelerate cutaneous aging according to at least one widely-accepted in vitro criterion, the reduction of culture lifespan for cells derived from the skin. Equally important for those committed to studying, modifying, or preventing actinically-induced aging, these data demonstrate that existing methodology can quantitatively assess subtle environmental effects on human skin. REFERENCES (1) V. J. Selmanowitz, R. L. Rizer and N. Orentreich, "Aging of the skin and its appendages," in Handbook of the Biology of Aging, Edited by C. E. Finch and L. Hayflick (van Nostrand Reinhold Co., New York, 1977) pp. 496-509. (2) B. A. Gilchrest, Relationship between actinic damage and chronologic aging in keratinocyte cultures of human skin,J. Invest. Dermatol., 72, 219-223 (1979). (3) B. A. Gilchrest, Prior chronic sun exposure decreases the lifespan of human skin fibroblasts in vitro, J. Gerontol., 35, 537-541 (1980). (4) L. Hayflick, The limited in vitro lifetime of human diploid cell strains, Exp. Cell Res., 37, 614-636 (1965). (5) V.J. Cristafolo, "Animal cell cultures as a model system for the study of aging," in Advances in Gerontological Research, B. L. Strehler, Ed. (Academic Press, New York, 1972). (6) L. Hayflick, Aging under glass. Exp. Gerontol., 5, 291-303 (1970). (7) G. M. Martin, C. A. Sprague and C.J. Epstein, Replicative lifespan of cultivated human cells. Effects of donor's age, tissue, and genotype, Lab. Invest., 23, 86-92 (1970). (8) S. Goldstein, Lifespan of cultured cells in progeria, Lancet, 1,424 (1969). (9) B. S. Danes, Progeria: A cell culture study on aging,J. ½lin. Invest., 50, 2000-2003 (1971). (10) s. Goldstein and E.J. Moerman, Chronologic and physiologic age affect replicafive lifespan of fibroblasts from diabetic, prediabetic, and normal donors, Science, 199, 781-782 (1977). (11) J. G. Rheinwald and H. Green, Serial cultivation of strains of human epidermal keratinocytes: The formation of keratinizing colonies from single cells, Cell, 6, 331-344 (1975). (12) L. Hayflick, The longevity of cultured human cells,J. Amer. Geriat. Soc., 22, 1-12 (1974). (13) J. A. Parrish, R. R. Anderson, F. Urbach and D. Pitts, UV-A.' Biological Effects of Ultraviolet Radiation with Emphasis on Human Responses to Longwave Ultraviolet (Plenum Press, New York, 1978). (14) R. Jackson, Solar and senile skin: changes caused by aging and habitual exposure to the sun, Geriatrics, 27, 106-112 (1972). (15) S. Shuster, M. M. Black and E. McVitie, The influence of age and sex on skin thickness, skin collagen and density, Brit. J. Dermatol., 93,639-643 (1975). (16) J. w. Eveson and D. G. MacDonald, Quantitative histological changes during early experimental carcinogenesis in the hamster cheek pouch, Brit. J. Dermatol., 98, 639-644 (1978). (17) G. E. Milo, Jr. and J. A. DiPaolo, Neoplastic transformation of human diploid cells in vitro after chemical carcinogen treatment, Nature, 275, 130-132 (1978). (18) E. Urbach, J. H. Epstein and P. D. Forbes, "Ultraviolet carcinogenesis: experimental global, and genetic aspects," in Sunlight and Man, T. B. Fitzpatrick, et al. Eds., (University of Tokyo Press, Tokyo, 1974)pp. 259-283.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)