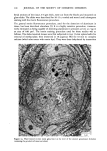
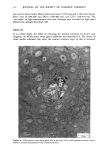
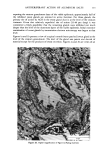
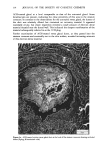
MELANIN OF HUMAN HAIR 149 VIII). Thus, the Irish red hair pigment is the richest in sulfur, while Scandinavian blond hair pigment has a low sulfur content. Table VIII Carbon/Sulfur Ratios (C/S) of Human Hair Samples Source of Pigment C/S Italian Brown Hair 13.4 Japanese Black Hair 15.7 Irish Red Hair 8.8 Scandinavian Blond Hair 35 C. STUDY OF ASSOCIATED PROTEINS We have analyzed protein materials after hydrolysis, by ion-exchange chromatography, using a Technicon TSM © analyzer. For hydrolyzing protein materials, most authors have used a method with distilled HCI in sealed test tubes at 110øC for 16 to 24 h. We already described, above, our specially equipped oven which permits agitation while samples undergo chemical hydrolysis with hot hydrochloric .acid. This method has the advantage of reducing to 4 h the necessary time of hydrolysis for the best yield of every aminoacid. Moreover, with such a method, some labile aminoacids can be recovered as, for instance, citrulline which is partly destroyed in a 24 h hydrolysis. When subjected to a 4 h hydrolysis, more than 85% of the citrulline can be recovered. I. External associated proteins. When we eliminate the last protein fractions from the raw undigested residue, using 3 hydrochloric hydrolyses, the first operation gives a typical keratin composition but the second and third hydrolysates show a different relative amino acid content. We call this associated protein "external," as opposed to the internal associated protein we find after solubilization of pure melanin. The external associated proteins obtained from various pigments are closely similar, and differ from hair keratinic protein (Table IX). They are particularly rich in leucine and valine. They contain more glycine and alanine, and much less dicarboxylic acids, proline, arginine and cystine than total hair. A small quantity of an amino compound that we attribute to citrulline (according to its position on chromatogram) is also present. The amounts of external associated protein vary according to the hair origin. For the Italian brown hair and for the Japanese black hair, the amount of external associated protein is about 7% based on the weight of pure melanin. This amount seems to be smaller in the case of Scandinavian or Irish hairs, but we could not measure it with precision. 2. Internal associated proteins. It is known that the solubilization of hair melanin can occur in alkaline hydrogen peroxide solutions (4). A 100 ml final aqueous solution, containing 2 ml of H20 2 (110 volumes), and 4 ml of NaOH 2N was prepared just before use. 500 mg of pure melanin were immersed in 70 ml of such oxidative solution for 30 minutes at 40øC.
150 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS Table IX Amino Acids Composition of External Associated Protei•a (Ex.A.P.). Units: 10 -2 Moles Human per 100 g Hair Total Protein (mean) Ex.A.P. Ex.A.P. Ex.A.P. Ex.A.P. From From From From Italian Japanese Irish Scandinavian Hair Hair Hair Hair Pigment Pigment Pigment Pigment Cysteic Acid 0.3 0.4 0.7 0.3 0.7 Aspattic Acid 4.5 1.5 1.4 1.0 1.6 Threonine 5.9 2.9 3.1 3.2 2.9 Serine 9.7 3.5 3.1 3.1 2.8 Glutamic Acid 10.1 2.7 1.8 2.0 2.2 Proline 6.5 1.4 0.9 1.7 1.5 Glycine 4.6 7.0 5.9 6.0 8.6 Alanine 3.5 3.9 4.5 6.0 6.0 Half Cystine 12.8 2.8 1.4 1.1 1.3 Valine 4.7 10.4 10.9 10.6 10.2 Methionine 0.5 0.5 0.5 0.2 0.1 Isoleucine 2.2 7.5 8.2 10.2 10.1 Leucine 5.1 19.1 17.5 20.6 18.3 Tyrosine 1.4 0.7 1.1 0.7 0.3 Phenylalanine 1.5 7.8 10.9 10.5 10.1 Lysine 2.0 2.4 2.9 1.6 1.9 Histidine 0.6 0.7 0.5 0.2 0.5 Arginine 5.3 1.7 1.3 0.3 1.7 Citrulline 0 0.6 0.7 0.1 0.4 The dark brown solution was centrifuged in order to remove small quantities of unsolubilized melanin. HC1 was added to the supernatant to adjust to pH 1 the oxidized pigment precipitated. Such pigment could be solubilized in an alkaline aqueous solution and precipitated when HCI was added. The solubilization precipitation cycle was repeated 4 times. All the acidic supernatants were mixed and analysed. Each type of pigment we studied, from Italian, Japanese, Irish or Scandinavian hairs, gave the same result. For 500 mg of pure melanin, we found 2.5 mg of protein, i.e. 0.5%. Subsequently, the melanin which had been solubilized at pH 9 was treated by proteinase PSF 2019 for 3 days at 30øC. Enzymatic hydrolysis removed again 0.5% of protein which has the same composition as the previous one. New enzymatic or HC1 hydrolysis does not remove any more proteinic materials. Internal associated proteins are similar for every kind of pigment (Table X). These proteins are specially rich in glycine. Cystine contents (that we have found in the form of cysteic acid, since the solubilization treatment oxidizes the major proportion of the disulfide bonds) are higher than in external associated proteins. They contain more dicarboxylic acids than external ones, especially aspartic acid. Citrulline is also present, as shown by the chromatograms. The quantity of sulfur present in the amino acid composition of internal associated protein is much less than that found in the pure melanins. We think that the sulfur atom is primarily incorporated in the melanin polymer. in one of our solubilization experiments with melanin derived from Italian hair, the IR spectra of sølubilized'reprecipitated melanin after removal of the 1% total protein
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)