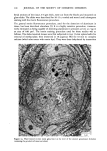
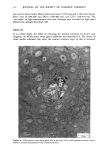
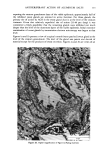
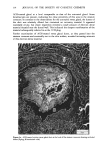
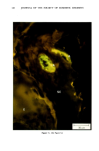
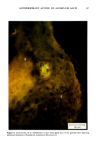
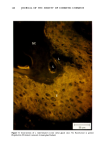
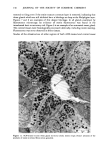
MELANIN OF HUMAN HAIR 139 4 days for the LiBr-sensitized hair, 1.8 g of purified lyophilised PSF 2019 was added to the solution at the end of 4 days, and the digestion was continued for 3 more days. At the end of the digestion, the insoluble residue (raw undigested residue) was filtered and washed with demineralized water until the supernatant liquid was neutral. 4. Purification. 1st hydrolysis. The raw undigested residue was refluxed in 500 ml of distilled HCI (-•5.6 N) during 4 hours in a l-liter flask. The insoluble residue was separated by centrifuging the acid solution. 2nd hydrolysis. Entire insoluble residue was suspended in 100 ml of distilled HC1 by mixing thoroughly and then distributed in 20 test tubes that were sealed and then placed in an oven at 100øC for 4 h. A special feature of the oven provided for the possibility of continuous agitation throughout the hydrolysis period. At the end of hydrolysis, the insoluble residue was separated by centrifuging the acid solution. 3rd hydrolysis. Exactly the same procedure as in the second hydrolysis was followed. At the end of hydrolysis, the insoluble protein-free pigment was separated by centrifuging, then washed with demineralized water by stirring and centrifuging until the supernatant liquid was neutral. It was then rinsed with ethanol and diethylether and allowed to dry at 40øC. III. RESULTS AND DISCUSSION In the initial phase of our work, we studied the different chemical methods previously reported for the isolation of melanin. It appears that all these methods have drawbacks. A. CRITICISM OF CHEMICAL METHODS I. Acid hydrolytic methods. LUBNOW (15) showed that when albino rabbit hair was hydrolized with hydrochloric acid, a brown insoluble product was formed. This melanin-like product can be obtained with all kinds of protein materials. When unpigmented proteins are hydrolized either under reflux with hydrochloric acid, or at room temperature for 20 days with hydrochloric or sulfuric acids, the solutions soon become yellow, green, then violet and brown. A dark brown product can be isolated. We obtained such products from gelatine, silk and different white hairs. We observed them under the electron microscope these products have no organized shape. In 1916, MAILLARD studied the formation of brown insoluble pigments when sugar and various amino acids or peptides are heated together (16). Such pigments have been examined by many authors (17-19). We found small amounts of reducing sugars in powdered hair or gelatine, using a method described for the analysis of carbohydrates in casein (20). Upon refluxing a hydrochloric acid solution containing a mixture of aminoacids (in the same relative composition of hair), we do not observe any coloration nor
140 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS precipitate. But if we add 0.2% of glucose, we observe a brown colouration and a progressive formation of melanoidic insoluble pigment.. So, the acid hydrolytic methods give rise to melanoidic artifacts which interfere with the isolation of natural melanin pigments. 2. Alkaline hydrolytic methods. The destructive effect of alkalies on aminoacids and more specifically on serine, threonine, cystine and cysteine, causes a possible transformation of the chemical composition of melanin pigments and associated proteins. We demonstrated this hypothesis, usiqg melanin prepared in vitro from dopa or dopamine with a mushroom tyrosinase, according to Mason (21). These melanins were treated either with 1 N sodium hydroxide solution at 37øC for 5 days, according to the Gjesdal method (11), or with a 2.5 N sodium hydroxide solution at room temperature for 12 h according to the Bold method (10). Together with the melanins, we treated a mixture of amino acids (in the same relative composition as that of hair) in the proportion of 0.2 g of melanin for 100 g of hair (approximate proportion of natural melanin pigment in brown human hair). We observed, first a partial solubility of melanin in alkaline solutions. Then, after washing the residue with water, acid solution, and ethanol, we analyzed it, and we found a small amount of sulfur. Thus, this element is coming from the degradation under hydrolysis of sulfur-containing amino acids (see Table I). Table I Sulfur Analysis of Treated Melanin Models Dopa-melanin Dopa-melanin treated together with a mixture of amino acids, with a 1N NaOH solution, at 37øC for 5 days Dopa-melanin treated together with a mixture of amino acids, with a 2.5N NaOH solu- tion, at room temperature for 12 hours Dopa-melanin treated together with a mixture of amino acids, with a solution of hyamine hydroxide at room temperature, for 4 weeks Dopa-melanin treated with the mixture phenol-thioglycolic acid at reflux, for 24 hours S = 0% S = 1.09% S = 1.12% S = 1.48% S = 1.58 3. Hyamine hydroxide method. Again some of the melanin pigment is partially dissolved in hyamine hydroxide solution. After partial solubilization, many of the decomposition products of cystine are incorporated in the melanin granules, as evidenced by the results of elementary sulfur analysis (see Table I). 4. PHT method. The chemical composition of natural melanin pigment is changed because small quantities of phenol and thioglycolic acid are introduced into the melanin structure. Using an original pyrolysing procedure (22), we found that when a pigment is isolated through the PHT method, the pyrolytic profile shows the presence of phenol. If we substitute in this method, orthocresol for phenol, the corresponding pigment gives the pyrolytic profile of cresol, although the pigment is heated under reflux with solvents of
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)