330 JOURNAL OF COSMETIC SCIENCE cosmetics and toiletry products sold in the year 2004 was $230 billion and continues to grow (2). Several online market researches anticipate the global cosmetic market to exceed $400 billion by the year 2027. In 2013, $407 million was spent on cosmetic and toiletry products imported into Malaysia, indicating that the demand for these products is mainly met by imports (3). This reflects both the undersupply and low consumer awareness of local cosmetic products. A study revealed that Malaysians are not aware of the cosmetic products made in Malaysia (4). Various active ingredients are included in cosmetic formulations for different functions, such as antioxidant activity, antimicrobial activity, antiaging properties, and UV filtering. However, not all active compounds are readily soluble in the solvents used in cosmetic formulations. Many techniques have been used to enhance solubilization of active ingredients in cosmetic formulations. These techniques include liposome, micelle, reverse micelle, solid lipid nanoparticle, noisome, nanocapsule, and others. Chemical stabilities, biocompatibility, and skin permeability of active ingredients can be increased with advance encapsulation technologies available for topical cosmetic application (5). The encapsulation technologies used in cosmetic formulations must also be non-irritating to the skin and nontoxic. Currently, in order to adhere to stricter regulations and consider an increasing public awareness of the environment, these techniques should also be sustainable and environmentally friendly. Amphiphilic molecules or surfactants can self-assemble into micelles (oil-in-water microemulsions) or reverse micelles (water-in-oil microemulsions) at appropriate compositions. They have unique structures that can be used to solubilize hydrophilic and lipophilic compounds. Aqueous solubility of hydrophobic compounds is greatly enhanced by encapsulation inside micelles, while oil solubility of hydrophilic compounds is greatly enhanced through encapsulation in reverse micelles. This characteristic is very useful for blending various active compounds in cosmetic formulations. Micelles are commonly used in cosmetic formulations due to many advantages, including ease of production, high encapsulation efficiency, protection of active ingredients, adjustable rheological properties, good skin-permeation properties, targeted delivery of active ingredients, and sustained release of active ingredients. However, there are fewer reports on the incorporation of reverse micelle in cosmetic products. This paper reviews the application of reverse micelles in cosmetics reported in the past two decades. The fundamentals of reverse micelle formation and the reports of reverse micelle for cosmetic applications are discussed in following sections. The authors’ intention is to gather as much knowledge as possible and provide insights on the topic. Reverse micelles have the advantages of normal micelles. However, due to the formulations of cosmetic products currently available, direct utilization of reverse micelles in the formulations is rare compared to normal micelles. Instead, most reports utilized reverse micelles for the extraction and purification of active compounds such as proteins and plant extracts for cosmetic formulations. More research on the use of reverse micelles for cosmetic applications is needed. ABBREVIATIONS: AOT Sodium bis(2-ethylhexyl) sulfosuccinate CMC Critical micelle concentration CTAB Cetyltrimethylammonium bromide
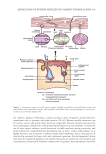
331 Application of Reverse Micelles in Cosmetic Formulations HA Hyaluronic acid HLB Hydrophilic lipophilic balance IL Ionic liquid IPM Isopropyl myristate ML Methyl laurate MO Monoolein PC Phosphatidylcholine SC Stratum corneum SURFACTANT Surfactants are one of the main components present in the mixture system aimed to form micelles. Surfactants are amphiphilic molecules that consist of both a hydrophobic tail and a hydrophilic head. The hydrophobic tail repels water or polar solvents while the hydrophilic head attracts them. The hydrophobic tail is made up of linear or branched alkyl chain lengths with 6 to 30 carbon atoms (6, 7). In general, surfactant molecules in a solvent will align themselves near the surface, or interfacial area, and reduce the surface or interfacial tension. The addition of surfactants into an oil and water mixture decreases the surface tension between the oil and water phases (8). Reduction of surface or interfacial tension then leads to micellization (9). This characteristic is exploited to greatly enhance the solubility of various molecules in aqueous or organic solvents. Surfactants can be classified as anionic, cationic, non-ionic, and zwitterionic according to the charge of their hydrophilic head groups (7). The charge on the surfactant head group determines the net charge of the micelles formed. It also affects the type and strength of interactions between the surfactant molecules and the solutes. In general, attractive electrostatic interactions are the main driving forces for the encapsulation of active compounds into reverse micelles. Nonetheless, hydrophobic interactions and hydrogen bonds also contribute to the encapsulation of active compounds. Some of the surfactants commonly used to form reverse micelles are anionic AOT and cationic CTAB. Zwitterionic surfactants may have a net positive, negative, or neutral charge depending on surrounding pH value, although they generally have a neutral charge. Lecithin is a commonly used zwitterionic surfactant in cosmetic formulations. Non-ionic surfactants are rarely used alone in reverse micellar systems due to their low encapsulation efficiency. However, their weaker interactions are beneficial in providing a milder environment for the better protection of the active ingredients. They are usually used together with ionic surfactants to form mixed reverse micelles. Some commonly used non-ionic surfactants are the Span series and Tween series. Surfactants have significant effects on the skin, such as altering the skin structure and allowing for the penetration of active ingredients. In order to maintain healthy skin or to avoid any clinical skin conditions, it is important to maintain the nature of the skin structure (10). Therefore, selection of suitable surfactants for the use in cosmetic formulations is crucial. The surfactants selected must not be skin sensitizers, irritants, or cause skin allergies. Sugar-based surfactants are recently applied in cosmetic products (11). These surfactants are milder to the skin while having good surfactant properties. Surface active ionic liquid (IL) has also gained increasing interest in cosmetic formulations. An
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)