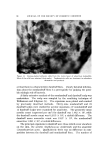
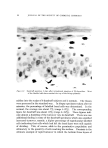
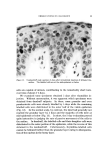
J. Soc. Cosmetic Chemists, 20, 135-152 (Feb. 5, 1969) Sorption of Ouaternary Ammonium Surfactants by Human Hair GEORGE V. SCOTT, Ph.D., CLARENCE R. ROBBINS, Ph.D., and JAMES D. BARNHURST, Ph.D.* Presented December 6, 1967, New York City Synopsis--The sorption of cationic surfactants (cationics) from aqueous solution onto hair is determined as a function of time using a solution depletion method. The solutions are periodically analyzed for cationic content by an Orange II dye procedure. Data from the sorption runs are conveniently processed by a programmed computer. Two quaternary amxnonium surfaetants, hexadeeyl-(CTAB) and dodeeyl-(DTAB) tri- methylammonium bromides, have been studied under various experimental conditions. The rate of CTAB sorption onto hair increases with increased pH or temperature, choice of buffer anion, or use of bleached hair. A decrease in "hair-to-solution" ratio also causes an increase in CTAB sorption. The rate of sorption decreases when lower solution concentra- tions are used or the eationic chain length is shortened (DTAB) Desorption of CTAB and DTAB is demonstrated by dilution of the partially depleted solutions in contact with hair. INTRODUCTION The action of quaternary ammonium surfactants is often dramatic on hair that has been damaged by treatments such as bleaching or cold waving. As creme rinses following a shampoo, they improve the hair with respect to feel or "hand," combing ease, snarl removal, and they de- crease electrical charges during brushing or combing. Quantitative tests are available in the literature which show that creme rinses reduce fric- tion (1), electrical charge buildup (2-4), and raspiness (5) of human hair. Attempted explanations of these actions invariably lead to considera- tion of the relative sorptive tendencies of these surfactants. Direct * Colgate-Palmolive Research Center, Piscataway, N. J. 08854. 135
136 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS information on sorption to hair, however, appears inadequate. Reese (6) reports results of a sorption study of quaternary and other ammonium salts to hair, but the longest hydrocarbon chain substituent, decyl, is shorter than those of commonly used surfactants. Other studies deal with substantivity to human hair of such diverse materials as amino acids sorbed from shampoo systems (7), acyl sarcosines (8), polypeptides derived from collagen (9), inorganic anions and cations (10), and phenols (11). Idson (12) has recently reviewed adsorption to hair (and skin) but reports no data for cationic surfactants. Much useful information on hair substantivity is obtained from the general subject of hair dyeing. Goldemberg (13) provides an interesting review and Underwood (14) discusses the basic factors involved in the sorption of dyes by hair. Wilmsmann (15) proposes that a barrier near the hair surface only admits dyestuffs whose average molecular diameters do not exceed about 6 •. This concept is considered further by Holmes (16) who uses diffusion constants determined for various dyes in water and in hair to estimate "pore size." Sorption of dyes is used by other authors (17, 18) to monitor the effect of hair treatments. Adsorption of Orange II anionic dye is determined by Rieger and Brechner (19) as a function of changes in pH, time, temperature, and other test conditions. They conclude with the justifiable assumption that the mechanism of hair dyeing is the same as that of wool dyeing. The similarity in chemical and physical behavior of the two keratin fibers aids hair research immeasurably in many areas. Dependence on information from wool research is clearly evident in this and other papers dealing with sorption on hair. To a major extent, this information de- rives from extensive studies of wool dyeing although reports on sorption of surfactants, generally anionic, are also available (20-24). The present objectives are to describe a procedure for determining sorption of cationic surfactants by hair and to discuss qualitatively the results for two surfactants examined under various experimental condi- tions. From a broader view, this paper will provide a background for comparison of commercial surfactants in a subsequent publication. EXPERIMENTAL MATERIALS AND METHODS The surfactants used are hexadecyltrimethylammonium bromide (CTAB), technical grade,* and dodecyltrimethylammonium bromide (DTAB), prepared at the authors' laboratories (% Br found, 26.01 cal- culated, 25.95). Stock solutions are prepared to contain approximately 2.4 mg solids per gram. * Eastman Organic Chemicals, Rochester, N.Y.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)