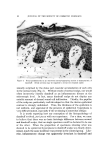
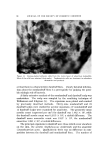
144 Figure 4. JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS • 2GM // õ •2 GM 5 tO 20 30 40 Effect of hair amount. Buffer at pH 3.6, citrate at 6.9, acetate tion. Results to be reported separately will show, however, that certain cationics of higher molecular weight than CTAB do penetrate more slowly, particularly at acidic pH. Effect of Change in Amount of Hair In the preceding experiments, approximately 1 g of hair was used for 60 ml of cationic solution. For experiments in the acidic range only, the use of a 2:60 ratio was investigated as a means of effecting larger concentration changes and thereby improving precision. The effect of this change on the sorption curves for CTAB at low and at neutral pH is shown in Fig. 4. On a "mg sorbed per gram of hair" basis, sorption on 2 g of hair is appreciably less than on 1 g. This fact is more evident from the data in Table III taken from the sorption curves at 24 hours. The sorption is less for the "2-g" experiments because the depletion of cationic from solution is greater and the curves tend toward lower equi- librium sorption. Effect of pH Change in the Acidic Range The pH range between 3.6 and neutral is of major interest from a formulation standpoint and it includes the isoelectric region of hair. Increasing the pH in this range causes the hair to change in volume (33, 35) and to shift from a positively-charged species to a noncharged species to a negatively-charged species relative to the liquid phase. It seemed desirable, therefore, to determine whether any abrupt change in sorption occurs in this range.
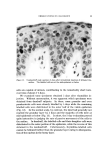
QUATERNARY SURFACTANTS ON HAIR 145 2O pH 64 5 10 20 30 40 (MINUTES) Figure 5. Effect of pH change in the acidic range. Hair, 2 g. Acetate buffers CTAB sorption to hair was determined at several pH values in the acidic range. Using 2 g of hair per run and acetate buffers, the results plotted in Fig. 5 were obtained. The data in Table IV are taken from the curves. It is interesting that the estimates of initial (one-minute) sorption by the fibers at each pH are appreciable fractions of the 24-hour values. This relates to the rapid pickup of cationic on sorption sites near the hair surface, followed by increasingly difficult penetration to interior sites. The one-minute sorption estimates indicate large changes in sorption with pH. The large change in sorption from pH 3.7 to 6.4 limits the 24-hour data for pH comparison since depletion of solutions increasingly depresses the curves as the pH is increased. Nonetheless, no abrupt changes in sorption were observed in this range. In practice, few basic dyes have been applied to wool and their be- havior is not too well known (33). At low pH, anionic dyes are con- sidered to sorb by an ion exchange mechanism to the positively charged fibers. At neutral pH, a leading ion mechanism is indicated wherein cations from the dyes are first attracted to the fiber and neutralize its negative charge, which makes it possible for the dye anions to approach and bind. By analogy, cationic surfactants may be expected to sorb to hair by a leading ion mechanism at low pH and by ion exchange at neutral pH. In an investigation of anionic dye sorption to wool as a function of pH, Deltachico and Peters (36) point out the basic similarity of the mechanisms for acid and neutral dyeing and show a smooth change of sorption from pH 4 to 9.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)