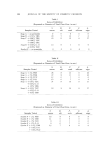
150 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS Table V Desorption Effects Mg/G of Hair % Conch. of Solution DTAB 24 hours sorption 4.80 0.0839 Dilution ... 0.0146 24 hours desorption 1.18 0.0190 CTAB 24 hours sorption 8.05 0. 0747 Dilution ... 0.0164 24 hours desorption 5.52 O. 0190 Effect of Hair Washed with Nonionic Surfactant Hair washed in a nonionic surfactant has been compared with hair washed in an anionic surfactant for sorption of CTAB, buffered at pH 3.6 with citrate. Both hair batches were similarly prepared and blended except for substitution of Tergitol ©* NPX for sodium lauryl sulfate. On the basis of single tests run in parallel, the nonionic washed hair sorbed less at each time interval. At 24 hours, for example, the values were 8.9 versus 10.4 mg CTAB per gram of dry hair. The influence of pre-sorbed sodium lauryl sulfate on the sorption of DTAB by hair has been studied in some detail and will be discussed in a [orthcoming paper. Effect of Hard Water Artificial hardness was tested for effect on CTAB sorption using 2 g of hair and pH 3.6 citrate buffer. The level of hardness was adjusted to 250 ppm as CaCO, using a mixed Ca-Mg salt solution. At this level of hardness, the molar concentration of divalent metal ions was about equiv- alent to that of the cationic surfactant. Sorption results were the same as those for previous runs without hardness. The metal cations thus do not compete effectively with cationic surfactants for the sorption sites of hair. Harry (44) described similar results pertaining to the influence of metal cations on Methylene Blue pickup. Reproducibility of Sorption Measurements Confidence in results is based mainly on the agreement of data from duplicate experiments. Additionally, replication has been tested in some instances for sorption runs separated in time. For the present cationics, such replication is represented by the experimental points for CTAB plotted in Fig. 8. * Union Carbide Corporation, 270 Park Ave., New York, N.Y.
QUATERNARY SURFACTANTS ON HAIR 151 SUMMARY Increase in sorption may be brought about by changes in pH, tem- perature, counterions, cationic chain length, and hair-to-solution ratio, and by damage to the fibers. Because the amount of cationic surfactant held at the hair surface is important, the sorption increases are con- sidered in terms of changes in affinity and changes in penetration rate. ACKNOWLEDGMENT Appreciation is expressed to Mr. D. McNeil for expert handling of the microscopical work and to Mrs. W. Bartok for care in obtaining much of the experimental data. (Received April 30, 1968) (10) (11) (12) (13) (14) (15) (16) (17) REFERENCES (1) Schwartz, A.M., and Knowles, D.C., Frictional effects in human hair, J. Soc. Cosmetic Chemists 14,455-63 (1963). (2) Mills, C. M., Ester, V. C., and Henkin, H., Measurement of static charge on hair, Ibid., 7, 466-75 (1956). (3) Levy, J. B., Wakelin, J. H., Kauzmann, W. J., and Dillon, J. H., Relation of charge to frictional work in the static electrification of filaments, Textile Res. J., 28, 897-911 (1958). (4) Barber, R. G., and Posner, A.M., A method for studying the static electricity produced on hair by combing, Y. Soc. Cosmetic Chemists, 10, 236-46 (1959). (5) Waggoner, W. C., and Scott, G. V., Instrumental method for the determination of hair raspiness, Ibid., 17, 171-9 (1966). (6) Reese, G., Adsorption of amines on hair keratin, Fette, Seifen, Anstrichmittel, 68, 763-5 (1966). (7) Herd, J. K., and Marriott, R. H., The sorption of amino acids from shampoos on to hair, J. Soc. Cosmetic Chemists, 10, 272-9 (1959). (8) Nelson, M. F., and Stewart, D., The adsorption of N-acyl sarcosines on various protein materials, Ibid., 7, 122-31 (1956). (9) Karjala, S. A., Williamson, J. E., and Karler, A., Studies on the substantivity of collagen- derived polypeptides to human hair Ibid., 17, 513-24 (1966) The effect of pH on the sorption of collagen-derived peptides by hair, Ibid., 18,599-608 (1967). White, H. J., and Underwood, D. L., The use of radiotracers to study adsorption by hair, Ibid., 7, 198-204 (1956). Breuer, M. M., Binding of phenols by hair, J. Phys. Chem., 68, 2067-73 (1964). Idson, B., Adsorption to skin and hair, J. Soc. Cosmetic Chemists, 18, 91-108 (1967). Goldemberg, R. L., Hair coloring--modern formulation considerations, Ibid., 10, 291- 306 (1959). Underwood, D. L., Basic elements of dyeing human h air, Ibid., 12, 155-62 ( 1961 ). Wilmsmann, H. Relation between high molecular weight aromatic compounds and their penetration for human hair, Ibid., 12,490 (1961). Holmes, A. W., Diffusion processes in human hair, Ibid., 15,595-608 (1964). Flesch, P., Protection of hydrogen peroxide bleached hair with acid solutions, Proc. Sci. Sect. Toilet Goods Assoc., 32, 1-5 (1959).
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)