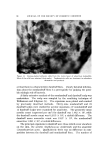
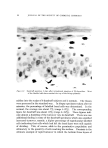
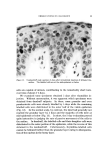
136 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS information on sorption to hair, however, appears inadequate. Reese (6) reports results of a sorption study of quaternary and other ammonium salts to hair, but the longest hydrocarbon chain substituent, decyl, is shorter than those of commonly used surfactants. Other studies deal with substantivity to human hair of such diverse materials as amino acids sorbed from shampoo systems (7), acyl sarcosines (8), polypeptides derived from collagen (9), inorganic anions and cations (10), and phenols (11). Idson (12) has recently reviewed adsorption to hair (and skin) but reports no data for cationic surfactants. Much useful information on hair substantivity is obtained from the general subject of hair dyeing. Goldemberg (13) provides an interesting review and Underwood (14) discusses the basic factors involved in the sorption of dyes by hair. Wilmsmann (15) proposes that a barrier near the hair surface only admits dyestuffs whose average molecular diameters do not exceed about 6 •. This concept is considered further by Holmes (16) who uses diffusion constants determined for various dyes in water and in hair to estimate "pore size." Sorption of dyes is used by other authors (17, 18) to monitor the effect of hair treatments. Adsorption of Orange II anionic dye is determined by Rieger and Brechner (19) as a function of changes in pH, time, temperature, and other test conditions. They conclude with the justifiable assumption that the mechanism of hair dyeing is the same as that of wool dyeing. The similarity in chemical and physical behavior of the two keratin fibers aids hair research immeasurably in many areas. Dependence on information from wool research is clearly evident in this and other papers dealing with sorption on hair. To a major extent, this information de- rives from extensive studies of wool dyeing although reports on sorption of surfactants, generally anionic, are also available (20-24). The present objectives are to describe a procedure for determining sorption of cationic surfactants by hair and to discuss qualitatively the results for two surfactants examined under various experimental condi- tions. From a broader view, this paper will provide a background for comparison of commercial surfactants in a subsequent publication. EXPERIMENTAL MATERIALS AND METHODS The surfactants used are hexadecyltrimethylammonium bromide (CTAB), technical grade,* and dodecyltrimethylammonium bromide (DTAB), prepared at the authors' laboratories (% Br found, 26.01 cal- culated, 25.95). Stock solutions are prepared to contain approximately 2.4 mg solids per gram. * Eastman Organic Chemicals, Rochester, N.Y.
QUATERNARY SURFACTANTS ON HAIR 137 Dark brown "blue string" hair* is cut into 1-in. lengths, washed with sodium lauryl sulfate solution, rinsed in water extensively, and dried at 50% RH, 24øC. Hand blending of the dry hair supplements wet blending accomplished during washing and rinsing in a laboratory wash- ing machine. The hair is stored in a closed container, protected from light, and is examined periodically for per cent volatile at 110 øC. Han- dling is similar for damaged hair but includes treatment for 40 minutes with a solution containing 6% hydrogen peroxide adjusted to pH 10 with ammonium hydroxide. Buffer stock solutions (0.2M) are prepared using sodium citrate- citric acid at pH 3.5, sodium acetate-acetic acid at pH 3.6, 4.6, and 5.7, ammonium acetate at pH 6.9, and sodium bicarbonate-carbonate at pH 9.3. Equipment used is commercially available and includes a thermo- stated shaker bath and preset automatic Bicknell pipets for sample with- drawals. Sorption Determination The procedure for determining sorption is as follows: Hair, weighed to the nearest mg, is added to a 125-ml glass stoppered Erlenmeyer flask with aliquots of buffer and water. The flask is immersed in a shaker water bath at 40.5 øC. Aliquots of cationic stock solution and buffer are added to a 50-ml volumetric flask, also placed in the bath. After warm- ing, the contents of the volumetric flask are transfered to the hair flask (zero time) and agitation is begun. At time intervals, withdrawals are taken from the hair flask with calibrated pipets and delivered into 25-ml Erlenmeyer flasks. Additions and withdrawals are each weighed to the nearest mg using the following quantities of reagents: Anticipated Sorption More than 25 Less than 25 mg/g hair •ng/g hair Hair flask Hair (g) 1 2 Deionized water (ml) 5 5 Buffer solution (ml) 5 15 Volumetric flask Cationic stock solution (ml) 25 25 Buffer solution (ml) 25 15 * De Meo Bros., 135 Fifth Ave., New York City.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)