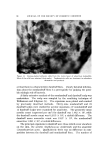
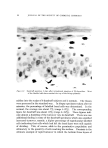
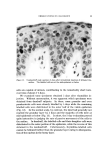
152 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS (18) (19) (20) (21) (22) (23) (24) (25) (26) (27) (28) (29) (ao) (31) (32) (33) (34) (35) (36) (37) (38) (39) (40) (41) (42) (43) (44) Laden, K., and Finkelstein, P., Studies concerning modification of ionic character of the hair, Am. Perfumer Cosmetics, 81, 39-42 (1966). Rieger, M., and Brechner, S., Studies on the adsorption of a simple dyestuff by hair, Proc. Sci. Sect. Toilet Goods Assoc., 34, 45-8 (1960). Steinhardt, J., and Zaiser, E. M., Combination of wool protein with cations and hydroxyl ions, J. Biol. Chem., 183, 789-802 (1950). Griffith, J. C., and Alexander, A. E., Equilibrium adsorption isotherms for wool/ detergent systems, J. Coll. Interf. Sci., 25, 311-21 (1967). Harris, J. C., Adsorption of surface-active agents by fibers, Textile Res. J., 18, 669-78 (1948). Swanston, K., and Palmer, R. C., The sorption of anionic detergents by wool, J. Soc. Dyers Colourists, 66, 632-8 (1950). Weatherburn, A. S., and Bayley, C. H., The sorption of synthetic surface-active com- pounds by textile fibers, Textile Res. Y., 22, 797-804 (1952). Scott, G. V., Spectrophotometric determination of cationic surfactants with Orange II, Anal. Chem., 40, 768-73 (1968). Speakman, J. B., and Smith, S. G., The structure of animal fibers in relation to acid dyeing, J. Soc. Dyers Colourists, 52, 121-35 (1936). Alexander, P., and Hudson, R. F., The kinetics of wool dyeing, Textile Res. J., 20, 481- 91 (1950). Medley, J. A., and Andrews, M. W., The effect of a surface barrier on uptake rates of dye into wool fibers, Ibid., 29, 398-403 (1959). Sagal, J., Acid and base binding behavior of white and pigmented human hair, Ibid., 35, 672-3 (1965). Steinhardt, J., and Harris, M., Combination of wool protein with acid and base, J. Res. Natl. Bur. Std., 24, 335-67 (1940). Ehrhardt, H., private communication. Vickerstaff, T., The Physical Chemistry of Dyeing, Interscience Publishers, Inc., New York, 1950, p. 119. Alexander, P., and Hudson, R. F., Wool--Its Chemistry and Physics, Reinhold Publishing Corp., New York, 1954, pp. 171-2, 240-2, 181. Valko, E. I., and Barnett, G., A study of the swelling of hair in mixed aqueous solvents, J. Soc. Cosmetic Chemists, 3, 108-17 (1952). Barnard, W. S., and White, H. J., The swelling of hair and a viscose rayon monofil in aqueous solutions, Textile Res. Y., 24, 695-704 (1954). Delmenico, J., and Peters, R. H., Application of the Donnan equilibrium to the distribu- tion of dye and inorganic ions between wool and solutions, Ibid., 35, 14-32 (1965). Alexander, P., and Charman, D. A., The kinetics of wool dyeing. Part II, The adsorp- tion of surface-active dyes by wool and other fibers, Ibid., 20, 761-70, (1950), Peters, R. H., Dyeing theories based on the latest research data, Ciba Rev. 1964• 2. Speakman, J. B., Stott, E., and Chang, H., Theory of milling II, J. Textile Inst., 24, 273-92T (1933). Zahn, H., Chemical processes in the bleaching of wool and human hair with hydrogen peroxide and peroxy acids, J. Soc. Cosmetic Chemists, 17, 687-701 (1966). Edman, W. W., and Marti, M. E., Properties of peroxide bleached hair, Ibid., 12, 133- 45 (1961). Klemm, E. J., Haefele, J. W., and Thomas, A. R., The swelling behavior of hair fibers in lithium bromide, Proc. Sci. Sect. Toilet Goods Assoc., 43, 7-13 (1965). Robbins, C. R., Infrared analysis of oxidized keratins, Textile Res. J., 37, 811-3 (1967). Harry, R., Modern Cosmeticology, Chemical Publishing Co., Inc., New York, 1962, p. 321.
Book Reviews REDUCTION: TECHNIQUES AND AP- PLICATIONS IN ORGANIC SYNTHESIS, Edited by Robert L. Augustine. Marcel Dekker, Inc., New York, 1968. 242 pages, indexed. Price $12.75. The proliferation of organic chem- istry reference text and methods books poses a difficult job of deciding when a book is a useful addition to a library and when the book is just another bound volume of superfluous information. Professor Augustine provides useful subject matter, brev- ity, and a relatively low price in his second volume on synthetic organic chemical procedures which make the book useful. The stated purpose is to provide concise and critical eval- uations of important synthetic re- actions for the practicing organic chemist who should be aware of all of the applications and suitability of a given reaction. While the title Reduction is too ambitious for the information contained therein, the book does go far in achieving its pur- pose. Three experts provide reviews which are comprehensive and, more importantly, are of direct utility in the laboratory. Professor Rerick's Chapter 1, "The Chemistry of the Mixed Hydrides," Dr. Smith's Chap- ter 2, "Dissolving Metal Reductions," and Professor Reusch's Chapter 3, "Deoxygenation of Carbonyl Com- 153 pounds" do creditable jobs of review- ing and interpreting the literature. The contributors include several good experimental procedures which are of immediate utility to bench chemists. One finds these procedures scattered throughout the book, but occasionally missing or insufficient in several places in Chapters 2 and 3. Sub- stantial reference sections are pro- vided at the end of each of the three chapters. Chapter 1, "The Chemistry of the Mixed Hydrides," discusses lithium aluminum hydride and sodium boro- hydride and their behavior in the presence of aluminum halides, metal salts, and alcohols to yield reducing agents of selected reactivities. Ex- perimental procedures are given for the reduction of a large number of functional groups as well as tech- niques and precautions for handling mixed hydride systems. The discussion of "Dissolving Metal Reductions" in Chapter 2 covers the recently developed metal- ammonia and metal-amine reagents as well as the older alkali metal- alcohol, metal amalgam-acid, and metal-acid systems. Experimental procedures described in this chapter are for the preparation of the reducing agent only literature references are cited for specific examples of reduc- tion. Chapter 3, "Deoxygenation of Car- bonyl Compounds," covers the Wolff-
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)