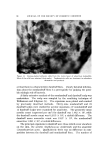
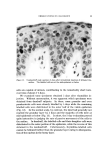
142 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS is most important with respect to hair properties such as static, friction, raspiness, ease of combing, and handling. As with wool dyeing (32), sorption of cationic surfactants from solu- tions to hair may be considered to involve three principal steps: (a) diffusion through the solution, aided by agitation, to the vicinity of the fiber/solution interface (b) adsorption at the hair fiber surface and (c) diffusion into the hair fiber and reaction according to affinity and number of sites available. The "ring dyeing" effect, seen in Fig. 2, indicates that reaction at internal sites is fast relative to diffusion within the fiber. Slow desorp- tion must also contribute to "ring dyeing" by retarding redistribution or leveling of the dye throughout the fiber (27, 33). Fiber diffusion is con- sidered in the present work as the slowest step, controlling the over-all sorption rate. Under other conditions, such as low concentration of sorbate, solution diffusion is reported as the rate-determining step (27). The pronounced sorption differences obtained for CTAB at different pH values may be attributed largely to changes in hair affinity with pH. At the isoelectric region of hair, internal neutralization of carboxylate and ammonium groups occurs. As the pH is raised, protons are re- moved from the ammonium groups and the carboxylate groups attract the cationic. As pH is lowered from the isoelectric region, carboxylate groups protonate and the ammonium groups repel the cationic. Change in fiber swelling with change in pH is important with respect to the rate of sorption of surfactants by the fiber. For immersed hair, a minimum volume exists at its isoelectric region (34, 35) and as hair equilibrates with solutions at higher pH fiber volumes increase. This permits cationic to diffuse faster into the hair. Thus, both the number and accessibility of sites within the fibers increase with pH to promote sorption. Effect of pH--DTAB The curves for dodecyltrimethylammonium bromide (DTAB)in Fig. 3 were obtained under conditions comparable to those for CTAB in Fig. 1 and qualitatively their appearance is similar. As with CTAB, equilibrium between hair and solution appears to have been reached at 24 hours at high pH. On this basis, the data in Table II indicate that CTAB has a stronger affinity for hair. The greater pickup of CTAB may result from increases in Van der Waal's attraction to the fiber and in partition tendency from water. Apparently, the longer hydrocarbon chain of CTAB is not an important factor with respect to hair penetra-
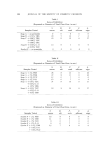
QUATERNARY SURFACTANTS ON HAIR 143 Figure $. pH 95 o 5 I0 20 30 40 (M•NUTES) I/2 Effect of pH on sorption of DTAB. Hair, lg. Buffer at pH 3.6, citrate at 6.5, acetate at pH 9.5, carbonate Table II Equilibrium Sorption of DTAB and CTAB (24 hours, pH 9.4) Micromoles % Solution Sorbed % Sorbed Cohen. DTAB 150 69.9 0. 029 CTAB 160 80.7 0. 020 Table III Effect of Change in Amount of Hair Grams Hair • Solution pH Mg Sorbed Per G Concn. % Depiction 1 3.6 13.5 0.086 18 2 3.6 10.4 0. 067 [/2 1 6.9 35.7 0. 054 48 2 6.9 21.0 0. 035 64 Table IV Effect of pH on CTAB Sorption in the Acidic Range Mg Sorbed/G of Hair Final pH 1 Min 24 Hr CTAB Analysis at 24 Hr % Conch. % Depletion 3.7 O.46 7.6 4.7 O.86 15.0 5.7 1.38 18.5 6.4 2.28 21.0 0.078 22 0.054 44 0.042 56 0. 035 64
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)