138 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS The amount of cationic solids in each withdrawal of 0.25 to 0.50 g of solution is determined by reaction with Orange II dye (25). From this, the cationic solids remaining in the bath and the cationic solids sorbing to the hair are calculated. Desorption Determination The procedure for determining desorption is as follows: After the last sorption withdrawal has been taken at 24 hours, most of the solution in contact with the hair is drawn off and replaced with buffer solution (0.1M) at 40.5 øC. The weight changes of the hair flask during the dilu- tion operation are determined and the amount of cationic remaining in the flask is calculated from the analysis of an initial desorption with- drawal and from the weight changes. Subsequently, periodic with- drawals of sufficient size are taken and analyzed with Orange II. Blank experiments were run with only hair and buffer and no cationic to determine whether material could be leached or shaken from hair (17, 19) which might interfere in the cationic assay. Withdrawals were taken from a citrate buffer solution after various times of shaking with hair. Acid and alkaline Orange II extractions of these withdrawals yielded no color in the chloroform layers. In a second experiment, a nonionic, Renex 30, added to carbonate buffer, was agitated with hair for 30 minutes. Again no color was obtained in Orange II extractions. Other blank experiments were run to determine whether, in the ab- sence of hair, loss of cationic might occur because of instability, sorption onto glass surfaces, or other causes independent of hair sorption. CTAB or DTAB have not been tested but two other quaternaries and an amine oxide have. Tests were conducted similarly to sorption runs, with omis- sion of hair. Withdrawals were taken in duplicate after overnight stand- ing of the solutions at 40.5 øC. The buffers used were alkaline carbonate and neutral acetate. Loss of cationics was 5% or less, averaging 3.4%. Since these amounts border on the precision limits of the analysis, all changes in the cationic concentration are ascribed to hair sorption. RESULTS AND DISCUSSION Sorption is determined by following the depletion of cationic sur- factant (cationic) from solutions in contact with hair. To improve the reliability of results, additions and withdrawals are determined gravi- metrically, and duplicate sorption experiments are run in parallel with alternating withdrawals. Calculations, based on cationic content and package directions for dilution of commercial rinses, indicate that 0.1% surfactant is a suitable
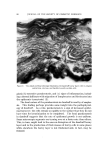
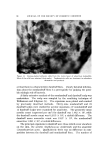
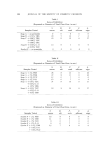
QUATERNARY SURFACTANTS ON HAIR 139 Table I CTAB Sorption--pH Effect Mg/G Hair Final pH 1 Min 24 Hours % Solution Depletion (24 hours) 3.5 O. 58 13.5 18 6.3 2.38 35.5 48 9.3 6.25 58 81 initial concentration. The depletion method, however, requires a much higher solution-to-hair ratio for the hair to remain completely immersed. Either of two ratios is employed depending on whether depletion is ex- pected to be large or small. In the former case, to avoid exhaustion of cationic, 60 ml of solution is used with 1 g of hair, and, in the latter case, to improve precision, 60 ml is used with 2 g. The amount (in mg) of cationic sorbed per gram of dry hair is plotted against the square root of time to test for the proportionality that is re- ported in dye sorption studies (19, 26-28) and to compress the larger time intervals. Effect of pH--CTAB Figure 1 shows the sorption of hexadecyltrimethylammonium bromide (CTAB) as a function of time from several buffered solutions and from one unbuffered solution. The need for pH control is apparent from the unbuffered run in which the pH decreased from 6.4 to 5.1. A compari- son of sorption at the several pH values is also shown by the data in Table I, abstracted from the graph. 60 pH 9• pH 6• 2O (MINtITEq) I/2 UNBUFFoeRoeD pH :•6 Figure 1. Effect of pH on sorption of CTAB. Hair, lg. Buffer at pH 3.6, citrate at 6.3, acetate at 9.3, carbonate. Initial pH of unbuffered run, 6.4 final pH 5.1
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)