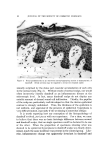
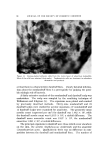
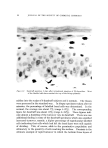
146 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS 20 Figure 6. "•1 I• 5 IO 20 $0 40 Effect of change in buffer anion. Hair, 2 g buffer pH 3.6 Buffer A nion Effect Sorption solutions are prepared with buffer at 0.13//concentration. Since the cationic surfactants are at a much lower concentration, sub- stantial interchange of anions must occur. Results for CTAB and DTAB are expressed as mg of the bromide sorbed per gram of hair al- though the buffer anion is involved. Practically, no problem arises in comparing results because the materials are on a common counterion basis. Aside from this aspect, a question arises in regard to the effect of buffer anions on cationic surfactant sorption. It should be noted that in the pH study in Fig. 1 different buffers were used. It was assumed that any specific buffer effect was minor relative to the pH effect. Specific buffer effects required more consideration when the citrate buffer had to be replaced with acetate at similar pH to avoid agglomeration of some higher molecular weight cationics. A comparison of these buffers for effects on CTAB sorption is shown in Fig. 6. Although initial sorption rates are similar, the curves diverge with time and show greater sorption with citrate buffer. The ionic weight of citrate is more than three times that of acetate. On the basis that penetration of hair by the quaternary salt would be easier with the smaller counterion, one might expect greater sorption with acetate. However, as in the comparison of CTAB and DTAB, this rule of thumb does not appear valid. Other factors must be dom- inant such as lower solubility of the quaternary citrate in water and a higher affinity for hair. In a practical sense, the counterions appear im- portant for a cationic formulation but their relative merits must be tested using the particular cationic surfactant.
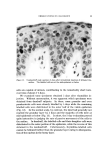
QUATERNARY SURFACTANTS ON HAIR 1-t7 25 • I0 140 *F ß • e o 5 105 øF I 5 IO 20 30 40 (MINUTES) I/2 Figure 7. Effect of temperature change. Hair, 2 g. Buffer pH 3.6, acetate Temperature Effect Sorption was determined in the preceding experiments at a temper- ature of 40.5øC. This temperature was selected as a reasonable es- timate for practical application of creme rinses. If the hair is heat dried, however, somewhat higher temperatures are encountered. Alexander and Charman (37) have observed that the resistance to washing of dye adsorbed on fabric is greatly increased by intermediate drying. This effect is probably caused by an increased tendency for adsorbed material to migrate into the fiber and to reduce the surface concentration. Con- sidering fiber diffusion as the rate-controlling step, this explanation im- plies that sorption rates will increase with temperature. CTAB sorp- tion was compared under similar conditions (2 g of hair, pH 3.6 acetate buffer) except for temperature. Results are shown in Fig. 7. From the curves, the estimates for "one-minute" and for "24-hour" sorption are 0.46 and 7.5 mg at 40.5 øC, 0.68 and 10.8 mg at 60 øC. The increase in CTAB sorption at the higher temperature is attributed mainly to easier penetration by the cationic, although other temper- ature-dependent factors must influence the rate. If temperature affects the rate of penetration only, the two curves should coincide at equi- librium. Peters (38) discusses this problem relative to fabric dyeing. The amount of dye taken up will increase with temperature provided that equilibrium is not reached in the dyeing period. However, for systems reaching equilibrium, higher temperatures decrease the uptake of dye. By analogy, the curves of Fig. 7 may cross before equilibrium is attained.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)