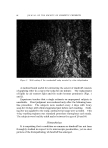
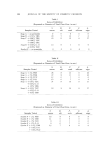
QUATERNARY SURFACTANTS ON HAIR 139 Table I CTAB Sorption--pH Effect Mg/G Hair Final pH 1 Min 24 Hours % Solution Depletion (24 hours) 3.5 O. 58 13.5 18 6.3 2.38 35.5 48 9.3 6.25 58 81 initial concentration. The depletion method, however, requires a much higher solution-to-hair ratio for the hair to remain completely immersed. Either of two ratios is employed depending on whether depletion is ex- pected to be large or small. In the former case, to avoid exhaustion of cationic, 60 ml of solution is used with 1 g of hair, and, in the latter case, to improve precision, 60 ml is used with 2 g. The amount (in mg) of cationic sorbed per gram of dry hair is plotted against the square root of time to test for the proportionality that is re- ported in dye sorption studies (19, 26-28) and to compress the larger time intervals. Effect of pH--CTAB Figure 1 shows the sorption of hexadecyltrimethylammonium bromide (CTAB) as a function of time from several buffered solutions and from one unbuffered solution. The need for pH control is apparent from the unbuffered run in which the pH decreased from 6.4 to 5.1. A compari- son of sorption at the several pH values is also shown by the data in Table I, abstracted from the graph. 60 pH 9• pH 6• 2O (MINtITEq) I/2 UNBUFFoeRoeD pH :•6 Figure 1. Effect of pH on sorption of CTAB. Hair, lg. Buffer at pH 3.6, citrate at 6.3, acetate at 9.3, carbonate. Initial pH of unbuffered run, 6.4 final pH 5.1
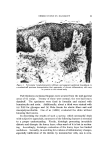
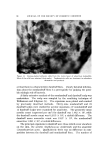
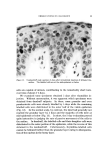
140 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS The amount sorbed in the first minute is estimated from the curves by drawing a line from the origin to coincide with the initial part of the curve. With the present experimental set-up, determinations made in the first several minutes of the sorption process were widely scattered. This is attributed to a rapid initial sorption and to mixing problems. The general shapes of the curves in Fig. 1, particularly in contrast to those for higher molecular weight cationics not reported, suggest that CTAB is diffusing into the hair fibers. The decreasing rate of cationic pickup over such a long period of time reflects the more difficult acces- sibility of sorption sites located further within the hair fibers. Only at high pH is there evidence that hair has reached equilibrium with solu- tion. At 24 hours, the molar quantity of sorbed cationic represents an appreciable fraction (ca. 35%) of the base-binding capacity of hair (29) or wool (30). It may be anticipated that this fraction will increase at a higher pH or a higher concentration of cationic. The change in sorption of CTAB as a function of time and pH led to the use of microscopy as a means of seeing how the cationic distributed itself inside the hair. White fibers, selected from gray hair, were ex- posed to buffered solutions of Methylene Blue, used as a colored cationic, under conditions similar to those for CTAB sorption. After contact with Methylene Blue, the fibers were cross-sectioned and photographed under the microscope (Fig. 2). With pH 3.6 citrate buffer for 60 minutes, the section shows little penetration of Methylene Blue. With pH 9.2 carbonate buffer for 60 minutes, the penetration is much deeper pH 9.5. This visual evidence for the dependence of cationic penetration on pH is limited by the extent to which Methylene Blue shares characteristics with cationic surfactants. For this reason another experiment was attempted. White fibers were treated with CTAB at high and at low pH for two hours at 40.5 øC. Cross sections of these fibers were stained under the microscope with a solution of Rubinc Dye,* an excellent in- dicator for long chain cationics that are sorbed on intact fibers (31). The stain, which remained after rinsing (Fig. 2), confirms the much deeper penetration of cationic surfactant under alkaline than under acidic conditions. It is estimated that 15-20% of the fiber radius is penetrated in two hours at high pH. Since the cuticle occupies approxi- mately 8% of the fiber radius, penetration into the cortex has already occurred. The need for information on the radial location of sorbed cationic is obvious when one considers that the cationic at or near the fiber surface * Direct Fast Rubinc WS, C. I. No. 28395, Ciba Company, Inc., Fair Lawn, N.J.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)