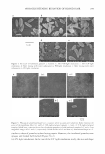
J. Cosnzet. Sci.! 55, 253-264 (May/June 2004) Ethosomes and liposomes as topical vehicles for azelaic acid: A preformulation study ELISABETTA ESPOSITO, ENEA MENEGATTI, and RITA CORTESI, Department of P harnzaceutical Sciences, University of Ferrara, I-44100 Ferrara, Italy. Accepted for publication April 6! 2004. Synopsis The basic properties and the in vitro release rate kinetics of azelaic acid (AA), alternatively vehiculated in different phospholipid-based vesicles such as ethosomes or liposomes, were investigated. Ethosomes were produced by a simple method based on addition of an aqueous phase to an ethanol solution (comprised between 20% and 45%, v/v) of soy phosphatidyl choline (5%, w/w) and AA (0.2%, w/w) under mechanical stirring. Liposomes were obtained by the same composition in the absence of ethanol with the reverse-phase evaporation method. Vesicle size was measured by photon correlation spectroscopy (PCS), evidencing smaller mean diameters and narrower dimensional distributions in the case of ethosomes with respect to liposomes. In order to obtain homogeneously sized vesicles, both ethosomal and liposomal dispersions were extruded through polycarbonate membranes with pores of calibrated diameter (400 nm and 200 nm). Vesicle morphology was characterized by freeze-fracture scanning electron microscopy (SEM) showing the presence of unilamellar vesicles both in liposome- and in ethosome-based dispersions. Free energy mea surements of the vesicle bilayers were conducted by differential scanning calorimetry (DSC). AA diffusion from ethosomal or liposomal dispersions and from ethosomes and liposomes incorporated in a viscous gel was investigated by a Franz cell assembled with synthetic membranes. The release rate was more rapid from ethosomal systems than from liposomal systems. In particular, ethosomes produced by the highest ethanol concentration released AA more rapidly, and the same trend was found using viscous forms. INTRODUCTION Azelaic acid (AA) is a saturated dicarboxylic acid found naturally in wheat, rye, and barley. It is a competitive inhibitor of mitochondrial oxidoreductases and of 5 alpha reductase, inhibiting the conversion of testosterone to 5-dehydrotestosterone (1). AA is an anti-keratinizing agent, displaying antiproliferative cytostatic effects on keratinocytes and modulating the early and terminal phases of epidermal differentiation. It also possesses bacteriostatic activity toward both aerobic and anaerobic bacteria, including Propionibacteriunz acnes (2). Acne is a skin disorder occuring when excess oil (sebum) production combined with dead skin cells clogs pores. Bacteria form in the pores, resulting in red, inflamed pimples, Address all correspondence to Elisabetta Esposito. 253
254 JOURNAL OF COSMETIC SCIENCE pus-filled whiteheads, or blackheads. There are many different forms of acne, but acne vulgaris is the most common form. The physiopathologic mechanism of acne seems to be dependent on four main factors: (a) sebum production and excretion, (6) type of keratinization of the follicular channel, (c) microbial colonization of the pilosebaceous unit, and (d) inflammatory reaction of the perifollicular area. AA is effective in the treatment of acne because it possesses an activity against all of these factors (3,4). AA is also efficacious in the treatment of alopecia aerata, a highly unpredictable autoimmune skin disease resulting in the loss of hair on the scalp and elsewhere on the body (1). In the present study the in vitro release rate kinetics of AA alternatively vehiculated in different phospholipid-based vesicles, such as ethosomes or liposomes, were investigated. Ethosomes could be described as lipid vesicular systems embodying ethanol in relatively high concentrations. These "soft vesicles" represent novel vesicular carriers for enhanced delivery to/through the skin (5 ). Ethosomes have a particle size that can be modulated from tens of nanometers to microns. One main feature of this new type of vesicle is its soft structure, which carries the incorporated active agent into the skin lipid bilayers, enabling facilitated delivery. The use of ethosomal carriers results in delivery of high concentrations of active to/through the skin, regulated by the system composition and their physical characteristics. Vesicle characterization can be assessed by DSC, DLS, NMR, ultracentrifugation, and electron microscopy. Dayan and Touitou (6) have dem onstrated the major potential of ethosomes to promote drug penetration through skin with respect to liposomes. In vivo experiments and clinical trials have demonstrated that a range of molecules such as testosterone, acyclovir (Zovirax Glaxo W ellcome pk), and insulin can be delivered effectively through the cell membranes of animal and human skin. An alteration of the ethosome formulation can modulate the level of penetration (restricting drug delivery to the skin only, as required for herpes labialis treatment with Zovirax, or allowing full dermal penetration, as required for insulin therapy) (7). Another molecule, trihexyphe nidyl hydrochloride, incorporated in ethosomes, is proposed for transdermal adminis tration in Parkinson patients, from which the geriatric population may greatly benefit (6). Transdermal absorption of polypeptides is currently under investigation (8). The high interest in ethosomes in the design of new therapies has been investigated with other drugs such as propranolol in this respect ethosomes have shown their potential as transdermal dosage forms for prophylaxis of migraine (9). Moreover, the ability of ethosomes to deliver compounds to cells in culture was investigated (10). The enhanced delivery of actives using ethosomes over liposomes can be ascribed to an interaction between ethosomes and skin lipids. A possible mechanism for this interac tion has been proposed. It is thought that the first part of the mechanism is due to the "ethanol effect," whereby intercalation of ethanol into intercellular lipids enhances lipid fluidity and decreases the density of the lipid multilayer. This is followed by the "ethosome effect," which includes interlipid penetration and permeation by the opening of new pathways due to the malleability and fusion of ethosomes with skin lipids, resulting in the release of the drug in deep layers of the skin (6). The purposes of this paper are (a) the production of ethosomes and liposomes as vehicles for azelaic acid (6) the characterization of the vesicles by freeze-fracture scanning elec tron microscopy (SEM), photon correlation spectroscopy (PCS), and differential scanning
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)