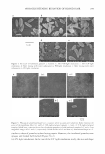
288 JOURNAL OF COSMETIC SCIENCE SDS aqueous solution (Figure 26), and in the 0/W microemulsion (Figure 2c) after a few hours. The AAPG complex seems to be more stable in the bicontinuous system (Figure 2d) and in the W/0 microemulsion (Figure 2e). The experimental results described above are consistent with our earlier statement (13), that the molecular complex between AA and PG is more stable in W/0 microemulsions. After a few hours the spectra a-c in Figure 2 look like a propy l gallate spectrum in the respective micellar system (similar to those used for preparing Figure 1). After a long time the bands characteristic of propyl gallate oxidation products at the wavelength about 260 nm are seen. The spectra in Figure 2d and 2e show how stable the AAPG complex is in an environment with a higher oil content (the bicontinuous system and the W/0 microemulsion). At the spectrum presented in Figure 2f (AA and PG in a mixture of 10% water and 90% pentanol), one can see three bands: two characteristic of propyl gallate ("-maxl = 217 nm and "-max2 = 273 nm) and one ("-max =265 nm) characteristic ,of ascorbic acid. The conclusion is that the kinetics of the AAPG complex formation in the system is very slow, but at the same time fast oxidation of vitamin C takes place (3). CONCLUSIONS 1. Ascorbic acid (AA) and propyl gallate (PG) become dissolved/solubilized in the microemulsion region of the SDS/pentanol/water system. 2. The results of the determination of propyl gallate kinetic decomposition/oxidation show faster oxidation of PG with increasing pentanol concentration in the micellar system. 3. The molecular complex between ascorbic acid and propyl gallate is formed in water, in surfactant aqueous solution, and in micellar systems. The complex created is the most stable in the W/0 microemulsion. 4. In the mixture of 10% water and 90% pentanol, the kinetics of AAPG complex formation is very slow, but at the some time fast oxidation of vitamin C takes place. REFERENCES (1) G. Guerin and A. M. Bellocq, Effect of salt on the phase behaviour of the ternary system water pentanol-sodium dodecyl sulfate, J. Phys. Chem., 92, 2550-2557 (1988). (2) S. E. Friberg, Ch. Brancewicz, and D. S. Morrison, 0/W Microemulsion and hydrotropes: The cou pling action of a hydrotrope, Langmuir, 10, 2945-2949 (1994). (3) M. Szymula, J. Szczypa, and S. E. Friberg, A comparison of atmospheric and electrochemical oxidation of vitamin C in SDS system,]. Dispers. Sci. Technol., 23, 1-9 (2002). (4) Y. K. Han, S. G. Oh, S. I. Shin, W. D. Joung, S. C. Yi, and C. G. Cho, Stability of alkanoyl-6-0- ascorbates in various surfactant aggregates systems, Colloid Surf B, 24, 33-44 (2002). (5) M. Szymula, Atmospheric oxidation of vitamin C and E in the surfactant system, J. Dispers. Sci. Technol., 21, 983-998 (2000). (6) M. Szymula and S. Radzki, A study of molecular complex formation between propyl gallate and ascorbic acid in the microemulsion phase of sodium dodecyl sulfate, pentananol and water, Colloids Surf B, 35, 249-257 (2004). (7) M. Szymula, Antioxidants activity in oil-i.n-water microemulsion stabilized by anionic surfactant, Anna/es Universitatis Mariae Curie-Sklodowska, Sectio AA Chemia, LVII, 271-280 (2002). (8) M. Szymula and J. Narkiewicz-Michalek, Atmospheric and electrochemical oxidation of ascorbic acid in anionic, non-ionic and cationic surfactant system, Colloid Polym. Sci. 281, 1142-1148 (2003). (9) K. Schwarz, S. H. Huang, J. B. German, B. Tiersch, J. Hartmann, and E. N. Frankel, Activities of antioxidants are affected by colloidal properties of oil-in-water and water-in-oil emulsions and bulk oils,]. Agric. Food Chem., 48, 4874-4882 (2000).
PROPYl GALLATE/ASCORBIC ACID ANTIOXIDATION 289 (10) H. Stockmann and K. Schwarz, Partitioning of low molecular weight compounds in oil-in-water emulsions, Langmuir, 15, 6142-6149 (1999). (11) Y. C. Chiu and W. L. Yang, Partition of vitamin E in microemulsion possessing high resistance to oxidation in air, Colloids Surf A, 63, 311-322 (1992). (12) Y. C. Chiu and F. C. Jiang, Determination of long-term stability of vitamin E emulsion and formation of microemulsion by using laser light scattering and UV absorption measurements, ]. Dispers. Sci. Technol., 20, 449-465 (1999). (13) M. Szymula, Atmospheric oxidation of J3-carotene in aqueous, pentanol, SDS microemulsion systems in the presence and absence of vitamin C,J. Dispers. Sci. Technol., 25, 129-137 (2004). (14) M. B. Davies, J. Austin and D. A. Partridge, Vitamin C: Its Chemistry and Biochemistry (Royal Society of Chemistry, Cambridge, 1991). (15) P. Becher, Dictionary of Colloid and Surface Science (Marcel Dekker, New York, Basel, 1990). (16) B. Katten, U. Beisiegel, G. Grecken, and A. Kontush, Mechanisms of lipid peroxidation in human blood plasma: A kinetic approach, Chem. Phys. Lipids, BB, 83-96 (1997). (17) M. Scarpa, F. Vianelleo, L. Signor, L. Zennaro, and A. Rigo, Inorgan. Chem., 35, 5201-5206 (1996). (18) K. Schwarz, E. N. Frankel, and J.B. German, Partition behaviour of phenolic compounds, Fett/Lipid, 98, 115-121, (1996). (19) V. A. Roginski, Effectiveness oflipid- and water-soluble phenolic antioxidants in the autooxidation of polyunsaturated fatty acids esters in microheterogenous solutions, Biol. Membr., 4, 437-451 (1990). (20) W. F. Linke, Ed., Solubilities of Inorganic and Metal-Organic Compounds: A Compilation of Solubility Data From the Periodical Literature, 4th ed. (American Chemical Society, Washington, D.C., 1965).
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)