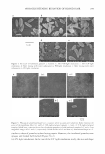
284 JOURNAL OF COSMETIC SCIENCE layer of the SDS molecule's association structures [the hydrated shell between the inner core and the polar heads of the surfactant molecules constituting the micelle (15 )}. Analyzing Figure 1, which presents the propyl gallate concentration changes (PG con centrations were calculated using absorbance values for the Amax2 band in various mi croenvironments), one can notice a similarity between ascorbic acid (3) and propyl gallate antioxidant behaviour. They are both good antioxidants in W/O microemulsions. The results of the kinetic determination of PG decomposition/oxidation in the micro emulsion show enhanced oxidation of PG with increasing pentanol concentration in the system. However, in this case the dependence of the oxidation rate on alcohol concen tration is less pronounced because the oxidation process is slower than for ascorbic acid (3 ). For both antioxidants the process is faster in the O/W microemulsion than in the SDS micellar solution. For the kinetic curves presented in Figure 1 we calculated the oxidation initiation rate R of propyl gallate according to R = 4-n 1 d [A0] 1 /dt, with n 1 as a stoichiometric factor characteristic of each antioxidant (bec�use of the lack of stochiometric factor data for propyl gallate, we took n = 2, as for ascorbate) and d [A0] 1 !dt as the consumption rate of each antioxidant. The rate R was calculated as a slope of linear regression of each experimental curve for all micellar systems (16, 1 7). The trend in the oxidation rate for propyl gallate changes in a systematic manner, i.e., in the W/O microemulsions the value of oxidation initiation rate R is higher than in the bicontinuous microemulsion and much higher than in the O/W system. In confrontation with the above data, we present our earlier results of the calculation of the parameter 0.002 i 0.0018 !e. � z 0 0.0016 z w u z 0.0014 0 u w 0.0012 CJ 0 ■ W-100,,, __.,_SDS8%,-94% -+-SDS 8%, water 93% • 0/W •·SDS8%,-88'11•0/W ·♦ SDS8%,-87%-0/W -+-SOS 8%, -72%•BC • SOS8",-55%-WIO W SOS8",-41%-WIO ♦ S0S8",-29'Jl•WIO SDSS'll,,-19"'-WIO .& SDS6%, ..... 1.,..,-W/O 0 naSDS.--10% 0 01W MK'ROEMULSION W..O MICROIEMULSION 0.001 -t------,-- - ----,---,------,-------,---.-----.---------.---,--- 0 2 3 4 5 6 7 8 9 10 TIME [weeks] Figure l. Propyl gallate (0.002% by weight) decomposition in the SDS/pentanol/water system. PG con centrations were calculated using absorbance values for the Amax2 280-nm band in various surfactant systems.
PROPYL GALLATE/ASCORBIC ACID ANTIOXIDATION 285 oxidation initiation rate R for ascorbic acid oxidation in various micellar systems (3) (Table II). The data presented distinctly shows that propyl gallate (as well as ascorbic acid) de composition is accelerated by a decrease in solvent polarity. It is well known that antioxidants possessing a hydrophilic character are, in general, less effective than lipo philic antioxidants in protecting lipids in 0/W emulsions. This fact can be explained in two complementary ways: First, from the discussions presented in the paper by Schwarz et al. (18), it follows that the lower activity of hydrophilic antioxidants in the 0/W emulsion than in bulk lipids can be attributed in part to partition of the antioxidants between the aqueous and the lipid phases. In emulsion the active proportion of polar antioxidants functioning as radical chain breakers is decreased due to the partitioning into the aqueous phase. Schwarz et al. (18) state that propyl gallate shows no activity or even functions as prooxidant in most emulsions, but exhibits high activity in bulk oil. Propyl gallate partitions partly into the aqueous phase, as indicated by their water-oil partition coefficient (P water-oil) of 0.895. Second, Roginski (19) suggests that the lower activity of antioxidants in emulsion might be also related to formation of H-bonded complexes between antioxidants and water molecules. The formation of H-bonded com plexes requires the presence of antioxidants at the water-oil interface or in aqueous phase. Thus, with increasing polarity, the formation of H-bonded complexes will rise. We should add to the above discussion that another factor that influences propyl gallate oxidation is higher oxygen solubility in non-polar solvents than in water (20). One can expect that with increasing pentanol, more oxygen will be dissolved in the system, causing acceleration of the oxidation reaction. In Figure 2 the changes in time of the representative spectrum of the mixture of AA and PG in various surfactant systems is shown. According to Figure 2 it is easy to notice that a molecular complex is formed when two antioxidants are present in water, in surfactant aqueous solution, and in the micellar systems studied. The formation of the AAPG molecular complex is discussed in detail in our previous paper (13). This complex decomposes in water (Figure 2a), in the 6% Table II Comparison Between Values of Propyl Gallate and Ascorbic Acid (3) Initiation Oxidation Rate R, Calculated From the Relation R = n [dC!dt} No. Micellar system R = -2 · dC!dt oxidation rate of propyl gallate (wt %/week) R = -2 · dC!dt oxidation rate of ascorbic acid (wt %/hr) 2 3 0/W microemulsion Bicontinuous microemulsion W /0 microemulsion -3. 10- 5 -4. 10- 5 -1 · 10-/4 0.00056 ± 1.34 · 10- 5 (SDS 6%, C5H110H 1 %, H20 93%) 0.0018 ± 6 · 10- 5 (SDS 6%, C5H 11 0H 22%, H20 72%) 0.00304 ± 6 . 10- 5 (SDS 6%, C5H110H 39%, H20 55%) Key: n is the antioxidant stoichiometric factor (for ascrobic acid, n equals 2 for propyl gallate, the n value is unknown, but n = 2 was taken in calculations). The derivative dC!dt was found from the linear regression experimental data: Dependence C = f(t), where C is the remainder of the antioxidant in the SDS/pentanol/ water system.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)