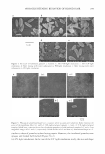
ETHOSOMAL AND LIPOSOMAL DISPERSIONS 263 The highest D coefficient was found in the case of ETHO 40, followed by ETHO 20. The more rapid release displayed by AA from ETHO 40 (9.99 cm/min°· 5 • 103 ) with respect to ETHO 20 (7.31 cm/min°· 5 • 103 ) can be attributed to the more fluid state of ETHO 40 vesicles, possibly promoting diffusion of AA through the vehicle. The D coefficient of AA from liposomes was approximately half the D coefficient from etho somes. Figure 3 reports a comparison between the release kinetics of AA solubilized in ethanol or ethosomal or liposomal dispersions and AA vehiculated in the same formulations viscosized in carbomer gels (namely EtOH carbomer gel, LIFO gel, ETHO 20 gel, and ETHO 40 gel). One should observe that the trend of AA diffusion from low-viscosity vehicles was confirmed when viscosized forms were studied, even if the incorporation of AA ethanolic solution or AA ethosomal or liposomal dispersions in carbomer gel re sulted in a slower release of the drug. This behavior can be attributed to the viscosity of the gel, which acts as an impediment to the release of AA. The lowest D .coefficient was found in the case of LIFO gel (1.9 cm/min°· 5 • 10 3 ). The D coefficient of AA from ETHO 40 gel was larger with respect to the D coefficient from EtOH carbomer gel. With regard to ethosomal dispersions, the presence of ethanol makes the lipid membrane less tightly packed than the conventional vesicles and confers a softer, more malleable structure to the ethosomes, possibly promoting AA diffusion through the vehicle. CONCLUSIONS The results here presented have demonstrated that AA-containing ethosomal vesicles, characterized by a morphology similar to that of liposomal ones, can be produced by a simple method. Extrusion of the dispersions allows one to obtain unilamellar vesicles of calibrated size. AA-containing ethosomes are characterized by a soft and malleable structure (as demonstrated by DSC studies) as a function of the ethanol concentration employed for their production. The more fluid state of ETHO 40 could be responsible for the higher AA diffusion through this vehicle with respect to ETHO 20 and LIFO. ACKNOWLEDGMENTS This work was supported by the Ministry of Education, University and Research ofltaly (MIUR) FIRB project. REFERENCES (1) A. S. Breatnach, Azelaic acid-Biological activities and therapeutic applications, Drugs Today, 25, 463-472 (1989). (2) Q. H. Nguyen and T. P. Bui, Azelaic acid: Pharmacokinetic and pharmacodynamic properties and its therapeutic role in hyperpigmentary disorders and acne, Int.]. Dermatol., 34, 75-84 (1995). (3) A. Fitton and K. L. Goa, Azelaic acid: A review of its pharmacological properties and therapeutic efficacy in acne and hyperpigmentary skin disorders, Drugs, 41, 780-798 (1991). (4) S. Passi, M. Picardo, C. De Luca, and M. Nazzaro-Porro, Mechanism of azelaic acid action in acne, Dermatol. Venereal., 124, 455-463 (1989).
264 JOURNAL OF COSMETIC SCIENCE (5) E. Touitou, N. Dayan, L. Bergelson, B. Godin, and M. Eliaz, Ethosomes-Novel vesicular carriers for enhanced delivery: Characterization and skin penetration properties,]. Controlled Release, 65, 403-418 (2000). (6) N. Dayan and E. Touitou, Carriers for skin delivery of trihexyphenidyl HCl: Ethosomes vs. liposomes, Biornaterials, 21, 1879-1885 (2000). (7) E. Horwitz, S. Pisanty, R. Czerninski, M. Helser, E. Eliav, and E. Touitou, A clinical evaluation of a novel liposomal carrier for acyclovir in the topical treatment of recurrent herpes labialis, Oral Surg. Oral Med. Oral Pathol. Oral Radio!. Endod., 87, 700-705 (1999). (8) E. Touitou, B. Godin, and C. Weiss, Enhanced delivery of drugs into and across the skin by ethosomal carriers, Drug Devel. Res., 50, 406--415 (2000). (9) P. Akkaramongkolporn, K. Terada, and E. Yonemochi, Molecular properties of propranolol hydro chloride prepared as drug-resin complexes, Drug Dev. Ind. Pharrn., 27, 359-364 (2001). (10) E. Touitou, B. Godin, N. Dayan, C. Weiss, A. Piliponsky, and F. Levi-Schaffer, Intracellular delivery mediated by an ethosomal carrier, Biornaterials, 22, 3053-3059 (2001). (11) F. Szoka and D. Papahadjopoulos, Procedure for preparation of liposomes with large internal aqueous space and high capture by reverse phase evaporation, Proc. Natl. Acad. Sci. USA, 75, 4194--4198 (1978). (12) L. D. Mayer, M. J. Hope, and P. R. Cullis, Vesicles of variable size produced by a rapid extrusion procedure, Biochirn. Biophys. Acta, 858, 161-168 (1986). (13) C. Nastruzzi, E. Esposito, C. Pastesini, R. Gambari, and E. Menegatti, Comparative study on the release kinetics of methyl nicotinate from topic formulations, Int.]. Phann., 90, 43-50 (1993). (14) R. L. Biltonen and D. Lichtenberg, The use of differential scanning calorimetry as a tool to characterize liposome preparations, Chern. Phys. Lipids, 64, 129-142 (1993). (15) C. Nastruzzi, E. Esposito, E. Menegatti, and P. Walde, Use and stability of liposome in dermatological preparations,]. Appl. Cosrnetol., 11, 77-91 (1993). (16) G. W. Y. Tin, Vesicle stabilization. Eur. Patent Appl. EP 162724 (1985). (17) K Thoma and U.E. Jocham, "Liposome Dermatics: Assessment of Long-Term Stability," in Liposorne Derrnatics, 0. Braun-Falco, H. C. Koning, and H. I. Maibach, Eds. (Springer-Verlag, Berlin, Heidel berg, 1992), pp. 150-166. (18) P. V. Shah, J. S. Elkins, J. Hanus, C. Noorizadeh, and J. P. Skelly, In vitro release of hydrocortisone from topical preparations and automated procedure, Pharrn. Res., 8, 55-59 (1991). (19) P. V. Shah, J. S. Elkins, and R. L. Williams, In vitro drug release measurements for topical gluco corticoid creams, Pharrnacopeial Forum, 19, 5048-5060 (1993).
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)