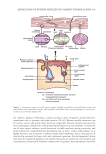
334 JOURNAL OF COSMETIC SCIENCE structure of normal micelles resembles core-shell structures where the core is composed of oil phase or hydrophobic solvent. Surfactant molecules align themselves so that their hydrophobic tails point toward the micellar core and their hydrophilic head groups at the micellar surface. The shape of micelle can be spherical or cylindrical depending on their p. The hydrophobic core can encapsulate hydrophobic molecules, thus significantly increasing their solubility in aqueous solutions. More hydrophobic molecules are generally located deeper inside the micellar core during encapsulation. Fewer hydrophobic molecules may solubilize near the oil-surfactant interface or in the palisade layer of the micelle. The structure of a spherical micelle encapsulating hydrophobic molecules is shown in Figure 1. The micellization process largely depends on the concentration of surfactant in the system (8). The number of micelles formed generally increases with surfactant concentration. High surfactant concentration may lead to micellar growth. The micelles formed are generally classified as spherical with their average diameter about double the diameter of the amphiphile surfactant molecule (6). Formation of three-dimensional, cubic micellar structures was reported when a high surfactant concentration is used (7). Temperature is another important factor affecting micellization and the structural morphology the micelles formed (16). This is related to the variation in the strength of the interactions between the surfactant molecules at different temperatures. Other factors such as surrounding pH, salt concentration, and the presence of additives may also influence the properties of the micellar system. Micelles can act as nano-sized carriers to transport active ingredients across skin. Hydrophobic active compounds can be delivered to a targeted spot by encapsulation within the micelles. The controlled release of active ingredients from the micelles can be achieved through proper formulation design (8). This allows delivery of active ingredients that are normally unable to penetrate the skin. In cosmetic applications, the size of the micelle is an important factor because only micelles with a smaller diameter than the pores of the Stratum corneum (SC) can penetrate the skin barrier, while larger micelles are obstructed from penetrating the skin (21). However, micelles do not necessarily need to penetrate the skin to deliver the active compounds. Micelles may disintegrate on the skin surface and in the hair follicles to release their contents into deeper skin layers. In cosmetic formulations, essential oils and hydrophobic active ingredients are added to skin care products, such as toner, through solubilization in micelles (9). Functions of the active ingredients in micelles are preserved and their stability can be improved, allowing the products to be stored for longer duration. Figure 1. Structure of a spherical micelle encapsulating hydrophobic molecules (7).
335 Application of Reverse Micelles in Cosmetic Formulations REVERSE MICELLE When surfactant concentration in an oil phase or nonpolar solvent reaches its CMC, reverse micelles are formed. The surfactant head groups gather to form an aqueous core while their hydrophobic tails point toward the bulk solvent phase. The structure of a spherical reverse micelle is shown in Figure 2. Size of reverse micelles are estimated through measuring the water content in the system (22). Karl-Fischer titration is usually conducted for measuring the water content of reverse micelle systems. Generally, the size of reverse micelles is from 1 nm to 10 nm (23). Cylindrical reverse micelles may also form depending on the composition of micellar systems and surrounding conditions. The water-to-surfactant ratio and the surfactant concentration in the bulk phase are important factors that determine the shape and size of reverse micelles formed (19). Regardless of shape, reverse micelles are very useful for the solubilization of polar compounds in nonpolar solvents. Reverse micelle systems usually contain a low amount of water. The water molecules are encapsulated in reverse micelles and dispersed in a nonpolar solvent (24). The polar compounds are encapsulated in the water cores of reverse micelles (17, 25). The suspension and dispersion of polar soluble ingredients in oil phases is made possible by utilizing reverse micelles formation (15). Reverse micelle systems are often used in the extraction of biological compounds, enhanced oil recovery, and the formation of nanoparticles. Reverse micelles are also used in the investigation of biomaterial due to their resistance to water properties (26). Reverse micelles can be produced at room temperature, thus making it more advantageous compared to other nanoparticle systems (27). Extraction using reverse micelles is usually done in two stages: the forward extraction and the backward extraction (28). Forward extraction involves selective dissolution of bioactive compounds into the reverse micelles, and backward extraction involves the release of encapsulated compounds into the fresh aqueous phase (22). Reverse micelle extraction is particularly effective at extracting charged compounds. The possibility of biodiesel production using diesel-based, reverse micelle was also reported (Nguyen et al., 2010). Reverse micelle systems have potential in formulating innovative cosmetic products. The following sections will focus on discussing the applications of reverse micelles in relation to the cosmetic field. Figure 2. Structure of a reverse micelle (43).
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)