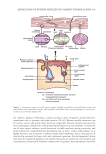
336 JOURNAL OF COSMETIC SCIENCE REVERSE MICELLE IN COSMETICS Reverse micelles can be applied in cosmetic formulations to encapsulate hydrophilic active ingredients. Encapsulation in reverse micelles can protect the active ingredients and preserve their activities. The solubilization and encapsulation of proteins and dyes in reverse micelles show potential improvements in cosmetic formulations (23, 29). Furthermore, the application of reverse micelles in cosmetic skin care products shows the enhanced penetration of active ingredients into the SC and through the epidermal barrier (17). In addition, reverse micelle extraction has been used to extract various active ingredients, such as plant extracts, for use in cosmetic formulations. DIRECT APPLICATIONS IN COSMETICS The direct inclusion of reverse micelles in cosmetic formulations is possible yet they are limited in their function. This means that reverse micelle systems may not be suitable for all skin care formulations. Substantial dirt such as sebum and cosmetic residues can be removed from the skin’s surface by using cosmetic products formulated with oil-based emulsion or microemulsion (9). Oil-based liquid cleansers have the advantage of retaining the oiliness on skin’s surface after cleansing. This is not achievable using typical water-based cleansers as they contain only a small amount of oil. This can be a reason for introducing reverse micelles into the cleansing formulations since the reverse micelles tend to form in nonpolar solvents such as oil (15). The reverse micelles in the formulations can improve the cleansing property through encapsulation. Examples of these cosmetic products are cleaning oils, gels, and lotions. Another cosmetic formulation that can benefit from using reverse micelles is lipstick. Lipsticks in general are made up of oil-based formulations which have a lower moisture content. To increase the moisturizing function of the lipsticks, reverse micelles are employed to encapsulate water-soluble collagen in the oil phase of the lipstick formulations (30). Reverse micelles allow hydrophilic active ingredients to be included in cosmetic formulations, especially the oil-based ones. The components used must be mild to the skin and nontoxic. Examples of such reverse micelle systems are AOT in orange essential oil (31), AOT in Isopropyl myristate (IPM), and AOT in Methyl laurate (ML) (32). These reverse micelle systems utilize nontoxic solvents and do not include co-surfactants. IL-AOT–based reverse micelle in IPM or ML shows potential in cosmetic applications (33). The advantage of IL is that it can be tailored to have certain properties, it has less toxicity, and it is environmentally friendly. The mixture of lecithin, sophorolipids, and rhamnolipids in IPM or ML is able to form reverse micelles at an appropriate mixture ratio (34). This reverse micelle system shows good tolerance toward changes in temperature and electrolyte concentration, thus is useful for designing more robust formulations. Inclusion of biosurfactants also helps improve the biocompatibility, sustainability, and biodegradability of the formulation. All of the aforementioned reverse micelle systems can be used to encapsulate hydrophilic active ingredients and are suitable to be used in cosmetic formulations. The encapsulation of various active compounds and drugs in reverse micelles has been reported. A food-grade lecithin reverse micelle system was used to encapsulate gallic acid, p-hydroxybenzoicacid, protocatechuic acid, and tyrosol (34). It was found that the antioxidant activities of the encapsulated active compounds are preserved. AOT/ Phosphatidylcholine (PC) and AOT/ Monoolein (MO) reverse micelles in soybean oil can solubilize ascorbic acid,
337 Application of Reverse Micelles in Cosmetic Formulations folic acid, and FeSO 4 (36, 37). PC acts as a zwitterionic co-surfactant, while MO acts as a non-ionic co-surfactant. The study showed that mixed surfactant systems can promote the formation of reverse micelles. The addition of NaCl may also promote the formation of reverse micelles. All components are food grade thus are safe to include in cosmetic formulations. Another study shows the encapsulation of acrylamide in AOT and lecithin reverse micelles. Micellar growth and sphere-to-cylinder transition were observed after the addition of acrylamide (38). Reverse micelle systems have been reported to solubilize various dyes. C-phycocyanin can be solubilized in AOT reverse micelles in isooctane (39). The resulting solution has a nice blue color appearance. This shows the potential to use natural dyes in cosmetic formulations through reverse micelle systems. Examples of reverse micelle systems encapsulating active compounds or drugs are given in Table II. The formation of cylindrical reverse micelles is triggered by adjusting the compositions of the reverse micelle system. When cylindrical reverse micelles become long enough to entangle each other, organogel is formed. The formation of organogel is mainly driven by hydrogen bonding and hydrophobic forces (49). Organogel still can effectively solubilize hydrophilic compounds in nonpolar solvents, but it has different viscoelastic properties compared to spherical reverse micelle systems. Lecithin is commonly used to form cylindrical reverse micelles. Lecithin alone forms spherical reverse micelles. Certain additives can trigger the sphere-to-cylindrical transition of lecithin reverse micelle and then lecithin organogel may form. A recent study shows that addition of sugar alcohols causes the formation of cylindrical lecithin reverse micelle in n-decane (50). The viscoelastic properties of the system can be controlled by using different sugar alcohols and temperatures. Another study shows that the addition of inorganic salts such as LiCl, LiBr, LiI, NaBr, NaI, and KI leads to the formation of lecithin organogel (51). The organogel was found to form easier when using alkane solvents with longer hydrocarbon chain length. The addition of certain carboxylic acids like citric acid and 1,2,3-propanetricarboxylic acid also caused the formation of lecithin organogel (52). It depends on the number of carboxyl groups in the carboxylic acids. Lecithin organogel has been tested for encapsulation of various active compounds such as methyl nicotinate, fenretinide, curcumin (53), caffeine or caffeine TABLE II Reverse Micelle Systems and Encapsulated Active Compounds or Drugs Reverse micelle system Encapsulated compounds Reference Lecithin/IPM Insulin 40 AOT/n-heptane Igepal CO-520/cyclohexane Ascorbic acid 41 Soybean phospholipid/camelina oil Quercetin 42 Glyceryl monooleate/IPM/isopropanol Hyaluronic acid 17 Reverse micelle form by FDA compliance materials Deferoxamine 43 Polyglyceryl-3-diisostearate/vegetable oils Polar antioxidants 44 Span 80/glycolipid/olive oil Diclofenac sodium 45 Food grade lecithin reverse micelle Gallic acid, p-hydroxybenzoicacid, protocatechuic acid, and tyrosol 35 lecithin/IPM Lidocaine 46 Tween 80/Span 20/IPM/isobutanol Sodium levothyroxine 47 AOT/PC/soybean oil Ascorbic acid, folic acid, and FeSO4 36 AOT/MO/soybean oil Ascorbic acid, folic acid, and FeSO4 37 AOT reverse micelles Lecithin reverse micelles Acrylamide 38 AOT/isooctane C-phycocyanin 39 AOT/IPM Glyceryl trinitrate 48
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)