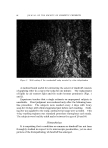
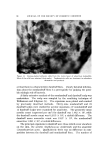
QUATERNARY SURFACTANTS ON HAIR 1-t7 25 • I0 140 *F ß • e o 5 105 øF I 5 IO 20 30 40 (MINUTES) I/2 Figure 7. Effect of temperature change. Hair, 2 g. Buffer pH 3.6, acetate Temperature Effect Sorption was determined in the preceding experiments at a temper- ature of 40.5øC. This temperature was selected as a reasonable es- timate for practical application of creme rinses. If the hair is heat dried, however, somewhat higher temperatures are encountered. Alexander and Charman (37) have observed that the resistance to washing of dye adsorbed on fabric is greatly increased by intermediate drying. This effect is probably caused by an increased tendency for adsorbed material to migrate into the fiber and to reduce the surface concentration. Con- sidering fiber diffusion as the rate-controlling step, this explanation im- plies that sorption rates will increase with temperature. CTAB sorp- tion was compared under similar conditions (2 g of hair, pH 3.6 acetate buffer) except for temperature. Results are shown in Fig. 7. From the curves, the estimates for "one-minute" and for "24-hour" sorption are 0.46 and 7.5 mg at 40.5 øC, 0.68 and 10.8 mg at 60 øC. The increase in CTAB sorption at the higher temperature is attributed mainly to easier penetration by the cationic, although other temper- ature-dependent factors must influence the rate. If temperature affects the rate of penetration only, the two curves should coincide at equi- librium. Peters (38) discusses this problem relative to fabric dyeing. The amount of dye taken up will increase with temperature provided that equilibrium is not reached in the dyeing period. However, for systems reaching equilibrium, higher temperatures decrease the uptake of dye. By analogy, the curves of Fig. 7 may cross before equilibrium is attained.
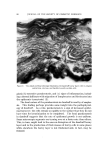
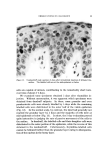
148 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS 20 Figure 8. ß 5 I0 20 $o 4.0 (MINUTES) I/2 Effect of hair bleaching. Hair, 2 õ. Buffer, 3.6 citrate The increase in penetration rate at higher temperature cannot be as- cribed to greater swelling of the fibers since temperature has little or no effect on the swelling of wool (39) or of hair (35) in water. Holmes (16) suggests that the high activation energy of diffusion is responsible for the temperature effects on sorption and that this energy reflects large changes in the viscosity of water within the fibers as temperature rises. Effect of Hair Damage Creme rinses owe much of their importance to the effects they pro- duce on hair that is damaged by bleaching or other abuses. The effect of bleaching treatment on CTAB pickup by hair may be seen in Fig. 8. The runs were each made using 2 g of hair and pH 3.6 citrate buffer. From the curves, the estimates for "one-minute" and "24 hour" sorption are 0.43 and 10.3 mg for undamaged hair, 1.88 and 15.8 mg for hair bleached 40 minutes with ammoniacal hydrogen peroxide. In a qualitative way, the increase in the rate of cationic sorption with oxidative damage to keratin fibers is rather well recognized (40). Solu- tions of Rhodamine and other cationic dyes have been used by beauti- cians and others to index the damage which hair has received and can withstand. Hair which becomes deeply stained under the test condi- tions is often referred to as "porous." Other indications of hair dam- age may be obtained from such measurements as tensile strength (41), swelling in aqueous media (42), alkali solubility, and cysteic acid con- tent (40). Aside from the desired bleaching of melanin, ammoniacal peroxide mainly attacks the disulfide bonds of crystine. For our purposes, this
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)