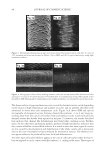
JOURNAL OF COSMETIC SCIENCE 204 observed that while shorter wavelength lasers can be used to obtain greater signal inten- sity, potentially reducing collection times, the shorter wavelength causes a large increase in background intensity, negating this benefi t. Exploratory experiments designed to determine the applicability of polarized Raman show changes in the spectra depending on orientation of the fi bers to the direction of the exciting beam. This may therefore offer potential to explore and estimate orientation of the crystalline intermediate fi laments in hair fi bers. REFERENCES (1) C. M. Pande. FT-Raman spectroscopy—Applications in hair research, J. Soc. Cosmet. Chem., 45, 257–268 (1994). (2) F. J. Wortmann, C. Popescu, and G. Sendelbach. Effects of reduction on the denaturation Kinetics of human hair, Biopolymers, 89, 600–605 (2008). (3) J. L. Haston, S. B. Engrlsen, M. Roessele, J. Clarkson, E. W. Blanch, C. Baldock, C. M. Kielty, and T. J. West, Raman microscopy and X-ray diffraction, a combined study of fi brillin-rich microfi brillar elasticity, J. Biol. Chem., 278(42), 41189–41197 (2003). (4) A. Kuzuhara, Analysis of structural changes in keratin fi bers resulting from chemical treatments using Raman spectroscopy, Biopolymers, 77, 335–344 (2005). (5) K. Schaefer. Natural fl uorescence of wool, J. Soc. Dyers Colours, 107(5–6), 206–211 (1991). (6) K. Song and J. F. Rabolt, Polarized Raman measurements of uniaxially orientated poly(ε-caprolactam), Macromolecules, 34, 1650–1654.
J. Cosmet. Sci., 60, 205–215 (March/April 2009) 205 A new oxidant for hair coloring JENNIFER MARSH, R. MARC DAHLGREN, COLIN CLARKE, JONATHAN STONEHOUSE, and CHRIS NUNN, Procter & Gamble, Miami Valley Innovation Center, 11810 East Miami River Road, Cincinnati, OH 45069 ( J.M., R.M.D.), and Procter & Gamble, Rusham Park Technical Centre, Whitehall Lane, Egham, Surrey, TW20 9NW, UK (C.C., J.S., C.N.). Synopsis Coloring hair using a level 3 permanent colorant involves two processes, lightening the underlying mela- nin and formation of the colored chromophores inside the hair. In typical in-market products the oxidant used to achieve these changes is hydrogen peroxide buffered at pH 10 with an alkalizer such as ammonium hydroxide. A new oxidant has been developed based on the combination of ammonium carbonate, hydrogen peroxide and glycine at pH 9 that can match the lightening and color performance of the current oxidant. It has the advantage that both the carbonate and hydrogen peroxide concentrations can be changed to alter the lighten- ing performance making it a more fl exible oxidant. This allows the capability to lighten the hair in a shorter time, or with lower hydrogen peroxide levels. This paper discusses the key oxidizing species that are present in both systems and the mechanisms of mela- nin lightening. In addition, the lightening performance will be assessed as a function of time, pH, hydrogen peroxide concentration and carbonate concentration. The importance of glycine to the oxidant is also described along with a proposal for its mechanism of action. It has been demonstrated that the addition of glycine can control the undesired formation of carbonate radi- cals that can be generated from the oxidant. The control of these radicals enables the oxidant to deliver excel- lent lightening with no negatives in fi ber damage vs. conventional oxidants. INTRODUCTION Level-three permanent hair colorant products typically consist of three major compo- nents: the oxidant which is hydrogen peroxide, the alkalizer which is typically either ammonia or ethanolamine and the dye precursors (1,2). The fi nal pH of the system is between pH 9.8 and 10.3. The role of the oxidant is three-fold. It must decolorize the melanin pigment to lighten the underlying color of the hair. It must also bleach previ- ously deposited synthetic color in the hair and it must couple together the dye precursors to form the colored chromophores. It is this balance of melanin lightening vs. deposition of synthetic color inside the hair that gives the hair its fi nal color. The lightening is very important to coloring products to enable the consumer to achieve the shade that she de- sires. This is critical for the blonde shades where very little dye is deposited and most of the fi nal color is due to the melanin lightening. However, it is also very important for
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)