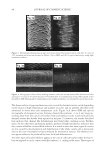
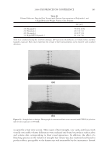
2008 TRI/PRINCETON CONFERENCE 89 defects approximately 1–3 μm in diameter form cavities fi lled with air as evidenced by their associated punctual bright colored spots due to light interference (see Figure 4a,b,c). An inspection of the SEM micrographs and the light interference patterns shown in Fig- ures 4a and 4b indicates that these types of defects are of two types, namely, (i) cavities forming bulges and blisters protruding away from the surface, and (ii) cavities buried deep beneath the surface with no bulging. The formation of such disruptions in the continuity of the cuticle cell certainly constitutes a loss in the cross-linking density of the protein structure due to a rapid expansion of water within the cuticle cell (see Figure 4c). GAPS AND CAVITIES FORMED BY SOLVENT EXTRACTION The immersion of hair fi bers in solvents increased substantially the average number of micro-cavities (see Figure 1, line c) although, not all cuticle cells were affected by the immersion and only a few cuticle cells underwent cavity formation. Furthermore, it was observed that immersion in IPA always led to higher increments in the number of micro- cavity and gap formation than did fi ber immersion in hexane. Increments in the number of gaps and cavities appearing in regions closer to the root after solvent immersion were low, while in regions away from the root, the increments were quite large. These observa- tions suggest that increments in the number of micro-cavity and gap formation observed after solvent immersion are related to damage that has been done to hair prior to solvent immersion. If this were not the case solvent immersion would produce equal increments of defects all along the fi ber from root to tip. However, before adopting this hypothesis as conclusive, we should analyze other alterna- tive explanations. One possible hypothesis is that gaps and cavities formed after solvent immersion is due to solvent swelling. For instance, it is known that IPA has the ability to swell the hair fi ber. However, hexane does not swell the keratin fi ber and yet it causes gap Figure 4. Micrographs (400×) of typical bulges and blisters formed in hair fi bers after exposure to fi ve cycles of 30 s in contact with a hot iron surface at 120°C followed by 30-s immersion in water. Figure 4a was ob- tained by SEM. Figure 4b, showing patterns of light interference, was obtained by optical microscopy. Figure 4c is a schematic representation of loss of continuity within the cuticle cell, leading to cavity formation by rapid water evaporation.
JOURNAL OF COSMETIC SCIENCE 90 and cavity formation, so the cause for their appearance must be found elsewhere. The other hypothesis is that the cavities may be caused by removal of endogenous lipids from cuticle cells since both solvents are known to extract lipids from hair. The nature of en- dogenous and exogenous lipids in hair has already been reported in the past (8). This latter hypothesis does not explain, however, why lipid extraction from cuticle cells only leads to the formation of gaps and cavities in a few cuticle cells and not in all cuticle cells. The endogenous lipids of hair exist only in the cell membrane complex and it has been reported that their removal requires high temperatures and long extraction times. One last hypothesis that might satisfactorily explain gap and cavity formation after sol- vent immersion is that these defects were already formed prior to solvent immersion and were later gradually fi lled with exogenous lipids, either from scalp sebum or from cos- metic formulations. In fact, GC-MS analysis of the material extracted from hair by sol- vent immersion indicated that it was mainly composed of exogenous lipids characteristic of sebum. The following fatty acids were found in the extracted material after methyla- tion: methyl caprylate, methyl laurate, methyl myristate, methyl palmitate, methyl li- noleate, and methyl stearate. These observations strongly suggest that gaps and cavities formed after solvent immersion were pre-existent to the analysis. The above described observations indicate that hair from common people contain gaps and cavities that can be classifi ed according to two main groups, namely: (a) gaps and cavities present in the cuticle sheath caused by damage done prior to the analysis that are visible by light interference, and (b) gaps and cavities that were also present before the analysis but that are not visible by light interference or by any other microscopic tech- nique due to the fact that they are fi lled with sebum and oils. These later types of gaps and cavities can be divided in turn into defects of natural origin, and defects produced by mechanical or thermal damage done to hair prior to the analysis. It should be mentioned here that gaps and cavities of natural origin were considered as those appearing in hair cut 2 or 3 mm away from the root and after solvent immersion. Because of their proximity to the scalp it is most likely that they were natural imperfec- tions in hair that were not caused by damage due to grooming stresses. They only ap- peared after exogenous lipids such as sebum were extracted from cuticle cells by solvent immersion. Therefore, their impregnation with sebum concealed their detection by both scanning electron microscopy and analysis of patterns of light interference. Inci- dentally, their count was extremely low and it was observed that not all fi bers had these natural defects. In contrast, the number of gaps and cavities that were concealed by sebum and appeared in hair sections away from the root after solvent immersion was always higher. It appears, thus, that sebum is able to wet and spread over the hair surface after it has been secreted from the sebaceous glands near the hair follicle. As the sebum spreads over the hair surface it is also able to penetrate and impregnate gaps and micro-cavities present in the cuticle sheath. This will explain why in many cases when hair fi bers obtained from common people were analyzed by optical microscopy, gaps and cavities were not detected until after the hair fi bers had been immersed in solvents. This explanation also implies that sebum diffused into gaps and cavities may not easily be removed by normal shampooing. Another observation confi rming that gaps and cavities were concealed by sebum inclusion and had formed prior to solvent immersion was that they vary in shape and size. Some of the gaps and cavities were of the extended type produced by extension and retraction stresses
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)