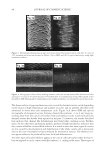
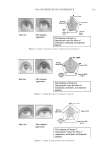
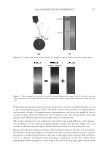
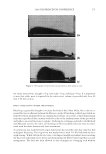
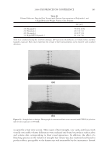
J. Cosmet. Sci., 60, 217–238 (March/April 2009) 217 Protection of oxidative hair color fading from shampoo washing by hydrophobically modifi ed cationic polymers Y. ZHOU, L. FOLTIS, D. J. MOORE, and R. RIGOLETTO, International Specialty Products, 1361 Alps Road, Wayne, N.J. 07470. Synopsis The fading of oxidative color in hair as a result of daily shampoo washing activities has become a com- mon problem and a source of frequent complaints by consumers. The fading occurs primarily through hair dye solubility in water. One aspect of the current study investigates the physical and chemical fac- tors that infl uence hair color fading during the washing process. This is accomplished by testing hair dye dissolution in water from dyed hair samples with variation of surfactant type, pH, and hair type. Furthermore, a new approach to preventing color fading is developed aiming to provide an effective barrier function for hair dye from dissolving into water. The preliminary investigation of a series of polymers with various functional groups indicates that polymers with hydrophobically modifi ed and cationic functionalities are most effective in preventing hair dye dissolution in water. It is also evident that a synergistic effect of the polymer’s hydrophobic moieties and cationic charges are important on hair color protection during shampoo washing processes. A primary example of a polymer within this category is a cationic terpolymer of vinylpyrrolidone, dimethylaminopropyl methacrylamide, and meth- acryloylaminopropyl lauryldimonium chloride (INCI: Polyquaternium-55). The color protection bene- fi t of this polymer is evaluated using newly developed methodologies for evaluating hair color changes, such as hair color fading tests through multiple shampoo washes with mannequin heads and hair tresses, both derived from human hair, colorimetry, and quantitative digital image analysis. In addition, new infrared spectroscopic imaging techniques are used to detect the hair dye deposition behavior inside hair fi bers both with and without the color protection treatment. Both visual and instrumental measurement results indicate that Polyquaternium-55 provides a high level of color protection when formulated in a hair color protection regimen with up to 50% color protection. This regimen signifi cantly outperforms commercial products that were tested containing a color protection claim. The proposed mechanism for the anti-fading action of hydrophobically modifi ed polymers includes a cationic charge-reinforced hy- drophobic barrier. This model is supported by evaluating the color fastness effect of several different polymer chemistries and by measuring hair surface hydrophobicity changes. INTRODUCTION Coloring hair becomes increasingly popular year to year. However, fading of artifi cial hair color has become a common problem and a source of frequent complaint by consumers. Lush, colorful hair begins to look dull with a loss of vibrancy and intensity, yielding a shade shift deemed non-desirable after a couple of weeks before roots grow out. The loss of dye hair color typically occurs because of color wash-out during the daily shampooing process or can be initiated by environmental circumstances such as exposure to UV radia- tion which can break down the color molecule. It was found that the washing process is
JOURNAL OF COSMETIC SCIENCE 218 the most signifi cant factor in the removal of hair color, while UV exposure has a signifi - cant impact only after 90 hours of intense irradiation (1). Though the hair color fading mechanism associated with the shampoo process is not yet well understood, there are some main factors attributed to fading. One is the dye solubil- ity in water. Most dyes used on hair are water soluble. Shampoo removal rate of dye is related to dye solubility. Dye solubility is related to dye structure and usually binuclear dyes are more soluble. Color fading is especially pronounced with red dye because it is more water soluble and is removed to a greater extent during washes, resulting in shade changes. In addition, the degree of hair damage has a signifi cant contribution to color fading through wash-out. Damaged hair is more porous which allows the dye molecules to leak out easily during the washing process. Therefore, bleached hair fades much faster than non-bleached hair because bleached hair is damaged with loss of F-layer protection and larger pore sizes on cuticle cells. Besides the pores on cuticle cells, the CMC and cor- tex swell with water, providing additional channels for dye to leach out. The surfactants in shampoo and conditioner products provide wetting function and bring moisture into the hair shaft which facilitates the dye molecules to come out with the water during washing. Although off and on-shade fading occurs during washing process, off shade fad- ing is much more objectionable by the consumer. Consumers want to maintain the vibrancy of the color until the next oxidative process. This translates to protecting hair color from fading for up to six weeks when color will need to be refreshed as noticeable re-growth will need to be colored. Meeting this con- sumer need continues to be a signifi cant challenge to the cosmetic industry. Color protec- tion is now considered to be an important area in hair care market (2–4). For the last couple of years, products for hair color protection have grown signifi cantly with over 170 color care products launched to the market globally (5). The majority of these products are in the form of shampoo and conditioners. Some of them use silicones which form a hydrophobic fi lm on the hair surface to protect color and there are several studies which have claimed the color retention effect by using this approach (1,6). The primary objective of our research was to understand the physicochemical factors af- fecting shampoo-caused off-shade fading and develop approaches that provide measur- able and consumer perceivable protection against color loss during shampoo washing of dyed hair. Our focus was to prevent color stripping of permanent, level 3, oxidative dye treated hair, as this comprises the majority of the professional and mass market formula- tions. To accomplish this objective, new methodologies for evaluating hair color change were developed and these methods and measurements were linked to consumer perceiv- able changes in hair color. With these methods, various types of polymers were tested for their effect on reducing hair color fading. An effective hair color protection treatment regimen containing a hydrophobically modifi ed cationic polymer was developed (patent pending). MATERIALS AND METHODS POLYMERS AND SURFACTANTS Polyquaternium-55 (Styleze W-20®) and other polymers tested in the dyed hair soaking tests, un- less specifi ed, were supplied by International Specialty Products (ISP). Alky hydroxy ethyl cellu- lose was supplied by Hercules. Quaternary salt of hydroxy ethyl cellulose was supplied by Rita.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)