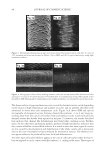
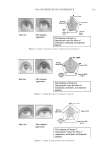
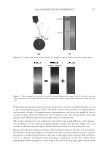
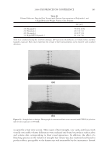
2008 TRI/PRINCETON CONFERENCE 237 (leave-in gel, shampoo and conditioner) and after 10× treatment and wash with a com- mercial color lock shampoo and conditioner system (from the bleached hair tresses tested in Figure 12). Again, the average water contact angle of PQ-55 anti-fading system treated hair fi bers is about 12 degrees higher than that of the commercial color-lock system treated hair fi bers, indicating the increased hydrophobic character of the hair surface after treatment with the PQ-55 system. Correspondingly, the treatment and washes by PQ-55 3 step anti-fading system provides 35% higher color protection than the commercial benchmark treatment as shown in Figure 12. The mechanism for absorption to keratinous substrates is a hydrophobic-driven and charge-driven process (9). The combination of hydrophobic substitution and cationic nature of PQ-55 allows the polymer to maintain good substantivity via its cationic units to color treated hair while provides a hydrophobic barrier to “lock-in” hair dye and pre- vent it from washing out during daily shampoo washing. The polymer also contains DMAPA unit which has a pseudo-cationic nature, providing additional substantive func- tion to hair. Figure 18 illustrates the proposed mechanism for color protection by Poly- quaternium-55. First, it adsorbs to the pores on cuticle surfaces. Some pores have more polymer deposition than the other. The polymer adsorbs around the pores, which leads to the complete sealing of the pores or a reduction in the size of these openings. Also, the polymer binds to the cuticle edges, which provides a protective layer on the cuticle ends. By blocking the two main open channels on hair surface by which dye molecules come out of hair, the polymer effectively protects hair dye being removed during wash. This mechanism can serve as a model for designing other polymer systems that will provide the color protection benefi t. CONCLUSIONS Effective polymer classes for hair color protection were identifi ed. Among them, Poly- quaternium-55 (PQ-55) demonstrated protection of oxidative color from shampoo stripping, exceeding the commercial benchmarks tested. The unique structure and functionality of Polyquaternium-55 has shown utility in helping to fulfi ll the current Figure 18. Proposed color protection mechanism for Polyquaternium-55.
JOURNAL OF COSMETIC SCIENCE 238 market need of oxidative hair color fastness from shampooing beyond its other hair care benefi ts such as conditioning and styling. The protective mechanism has been under- stood to be the combination of hydrophobic substitution and cationic nature of the polymer which forms a hydrophobic and substantive barrier fi lm on hair. The effective- ness of the protective benefi t of PQ-55 are substantiated from hair tress and mannequin heads made with human hair and have been quantifi ed with well known measurement techniques such as Hunter L,a,b and digital image analysis. The data from these tech- niques confi rm what is perceivable by the naked eye. In addition, measurements such as contact angle demonstrated the presence of the hydrophobic barrier formed by Poly- quaternium-55 on the hair surface and FTIR image analysis confi rmed the dye molecule retention improvement within the hair fi ber of Polyquaternium-55 treated hair. Multiple washing tests with various treatment regimens with formulas containing PQ-55 indicate that optimal effects are realized by applying a combination of leave-in and shampoo prod- ucts. The understanding of the structure–functionality relationship of PQ-55 opens the ways to the development of new anti-fading systems to meet the new market need of oxidative hair color fastness from shampooing. ACKNOWLEDGMENTS The authors would like to thank Xiaohong Bi for conducting FTIR image analysis Lynda Conforti-Tobin for conducting the Salon evaluation of mannequin heads Donna Laura and Grisel Tumalle for help in color fading tests Roger L McMullen for conducting digital image analysis and Solomon Wossene for help in Mannequin tests and for provid- ing subjective panel test results. REFERENCES (1) S. Marchioretto, The use of silicones as a color lock aid in rinse-off hair conditioners, J. Cosmet. Sci., 55(1), 130–131 (2004). (2) B. Brewster, Color lock in hair care, Cosmet. Toiletr., 121(3), 28–36 (2006). (3) C. Fox, Caring for color-treated hair, Cosmet. Toiletr., 120(11), 36–43 (2005). (4) G. Wis-Surel, Some challenges in modern hair color formulations, Int. J. Cosmet. Sci., 21, 37–340 (1999). (5) M. Reisch, Color protection, Chem. Eng. News, 30 (June 9, 2008). (6) A. Schlosser, Silicones used in permanent and semi-permanent hair dyes to reduce the fading and color change process of dyed hair occurred by wash-out or UV radiation, J. Cosmet. Sci., 55(Suppl.), S123– S131 (2004). (7) R. L. McMullen, J. Jachowicz, and Stephen P. Kelty, Correlation of AFM/LFM with combing forces of human hair, IFSCC Magazine, 3(3), 2–8 (2000). (8) C. LaTorre, B. Bhushan, J.-Z. Yang, and P. M. Torgerson, Nanotribological effect of silicone deposition level, and surfactant type on human hair using atomic force microscopy. J. Cosmet. Sci., 57(1) (2006). (9) C. Robbins, C. Reich, and A. Patel, Absorption to keratin surfaces: A continuum between a charge- driven and a hydrophobically driven process, J. Soc. Cosmet. Chem., 45(2), 85–94 (1995).
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)