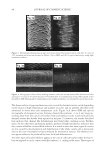
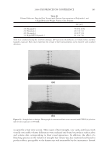
2008 TRI/PRINCETON CONFERENCE 225 solution was made using citric acid/citrate buffer, pH 6 solution was made using a phos- phate buffer, and pH 10 solution was made with a bicarbonate buffer. Figure 3 shows the color change of soaking liquors from soaking dyed bleached hair at three different pHs. The data indicates that hair dye dissolves in water much faster at a higher pH than at a lower pH. The faster dissolution of hair dye in water at a higher pH might be attributed to the known fact that, hair cuticle cells are open or swelled at a high pH. This allows more dye molecules to come out of the hair shaft. At a low pH, hair cuticle is contracted and intact, therefore allows less chance for the dye to come out of the hair. In addition, there is a larger difference between the color loss at pH 3 vs pH 6.5 or 10, especially during the initial soaking period. The pH 6.5 and 10 soaking has a similar hair dye dissolution pro- fi le. This can be explained by an increased pH in soaking liquor caused by alkalis inside the dyed hair which are from the hair dyeing process and can come out of the hair then go into the soaking liquor when hair is soaked in water. These alkalis can be neutralized more effi ciently in a low pH soaking liquor than in a neutral or a high pH soaking liquor. ANTI-FADING EFFECT OF HYDROPHOBICALLY MODIFIED CATIONIC POLYMERS Synergistic effect of hydrophobicity and quaternization. One of the approaches developed in this work for protecting shampooing-induced hair color fading is to minimize hair dye disso- lution in water by using a polymer barrier on hair surface. This polymer barrier is designed to prevent moisture from going in and out of hair which will reduce the chance for dye molecules to leach out of the hair shaft. Another approach is to develop a treatment regi- men containing the effective polymer to deliver consumer perceivable benefi t in leave-in treatments, stylers and shampoos. A number of polymers have been tested for their anti-fading effect. Test results of se- lected vinyl pyrrolidone copolymers containing various functional groups such as hydro- phobic unit and quaternary groups are reported here. The test polymer was formulated into a leave-in treatment gel formula and tested for their anti-fading effect in a 10× shampoo washing test against the same gel formula but without polymer in it. A leave-in treatment gel formula containing 2% active of Polyquaternium 55 is list in Table II. The chemical structures of the four polymers tested, including an anionic polymer for Figure 3. Effect of soaking liquor pH on hair dye dissolution in water, deep red colored hair, pH 5. Dyed bleached dark brown hair.
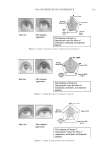
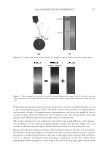
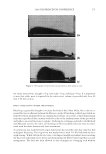
JOURNAL OF COSMETIC SCIENCE 226 comparison, are shown in Figure 4. Figure 5 shows the anti-fading test results of the four polymers in a leave-in treatment at 2% level using bleached hair tresses. The results demonstrate that Polyquaternium 55 (VP/DMAPA/C12-MAPTAC copolymer) leave-in treatment provides the highest color protection with 38% higher color improvement at the end of 10× washes over the control and the effect is well perceivable by eye as shown in the picture. VP/DMAPA acrylates copolymer which has a similar structure to Poly- quaternium 55 (PQ-55) but missing a hydrophobic chain and a quaternary group is less effective than PQ-55, with 17% color protection. Moreover, Polyquaternium-28 (VP/ MAPTAC), the quaternized analogue of VP/DMAPA acrylates copolymer without a hy- drophobic chain, is not effective on hair color protection. Therefore, the color fading test results of these three polymers demonstrate the synergistic effect of hydrophobicity and quaternary moiety on PQ-55. Consistently, the data in Figure 5 also shows that the hy- drophobically modifi ed anionic polymer, VP/acrylates/lauryl methacrylate copolymer provides negligible anti-fading effect due to lack of the cationic unit. Therefore this re- sult supports further a synergistic effect of hydrophobicity and quaternization of PQ-55 on hair color protection. The anti-fading effect of hydrophobically modifi ed quaternary salt of hydroxyl ethyl cel- lulose along with other HEC derivatives such as quaternary HEC and alky HEC were tested in a 10× wash color fading test. The results in Figure 6 show that alkyl substituted quaternary HEC provides higher color protection effect than the other two HEC deriva- tives which have no alky substitution or quaternary unit. These results demonstrate again the synergistic effect of hydrophobic and quaternary moieties of a polymer on hair color protection. The results in Figure 6 also show that PQ-55 is much more effective on hair color protection than the alkyl quaternary HEC tested. The higher color protection ben- efi t provided from PQ-55 might be partly attributed to the additional substantivity ef- fect provided by the DMAPA unit on PQ-55. Color fastness test results of PQ-55 tested in treatment regimens Pre-shampoo leave-in treatment with PQ-55. Dyed bleached hair was treated with PQ-55 leave in gel formulation (Table II) then dried before the fi rst shampoo wash. In a control test, the hair tress was treated with the same formula to which 2% PQ-55 was not added. 12% SLES was used as a washing agent for both tresses. The treatment, drying, shampoo washing cycle was repeated for 10 times. Figure 7a shows the total color changes of hair tresses determined as dE values after multiple treatments and wash cycles. The data shows that PQ-55 leave-in gel treatment provides 38% color protection after 10× washes over the control. Figure 7b shows the subjective panel test results of hair tresses after 10× wash and treatment from the test shown in Figure 7a. The 18 panelists agree that the hair tress treated with 2% PQ-55 leave in gel is signifi cantly darker than the hair tress treated Table II Anti-Fading Leave-In Treatment Gel Containing Polyquaternium-55 Ingredients %w/w Supplier Water 87.00 Hydroxpropyl guar (Jaquar HP-60) 1.50 Rhodia Polyquaternim-55 (20% active) 10.00 ISP Propylene glycol (and) diazoklidinyl urea (and) iodopropynyl butylcarbamate (Liquid Germall® Plus) 0.50 ISP Glycerin 1.00 1.00
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)