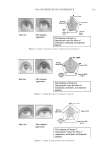
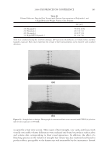
JOURNAL OF COSMETIC SCIENCE 212 Table III Lightening as a Function of Ammonium Carbonate and Hydrogen Peroxide Concentration Oxidant system Time Change in lightening (dL) 4.5% hydrogen peroxide/1.3% ammonia, pH 10 30 min 7.84 4.5% hydrogen peroxide/2.5% ammonium carbonate, pH 9 30 min 8.20 3% hydrogen peroxide/4.5% ammonium carbonate, pH 9 30 min 7.82 3.0 hydrogen peroxide/6% ammonium carbonate, pH 9 10 min 7.90 Table IV Lightening as a Function of Ammonium Carbonate and Hydrogen Peroxide Concentration Oxidant system Time Change in lightening (dL) 4.5% hydrogen peroxide/1.3% ammonia, pH 10 30 min 7.84 4.5% hydrogen peroxide/7% ammonium carbonate, pH 9 30 min 13.41 either with a different hydrogen peroxide level or with a different time using the same hydrogen peroxide level. For example, Table III shows different options with the new oxidant to match the lightening of a medium blonde product. (i.e. 1.0% ammonia and 3% hydrogen peroxide. The same level of lightening can be achieved using the ammo- nium carbonate, hydrogen peroxide and glycine oxidant in three different ways: (i) Equal hydrogen peroxide level. (ii) Lower hydrogen peroxide level. This may be an advantage for formulating shades where increasing hydrogen peroxide is not desired (e.g. for a low skin irritation pro- fi le or where regulations prohibit certain hydrogen peroxide levels). (iii) Shorter time (for example, matches the lightening but in 10 minutes vs. 30 minutes) An additional advantage of this lightening system is the ability to increase both the am- monium carbonate and hydrogen peroxide levels to increase the lightening beyond what can be achieved with the current oxidant. Table IV below shows the lightening for the two oxidants each with 4.5% hydrogen peroxide. (III) LIGHTENING AS A FUNCTION OF SOURCE OF CARBONATE (I.E., AMMONIUM CARBONATE VS. POTASSIUM HYDROGEN CARBONATE) The formation of the peroxymonocarbonate oxidant system does not depend on the pres- ence of ammonia to form the oxidzing species. However, when a cream formulation was tested with potassium hydrogen carbonate instead of ammonium carbonate the level of lightening was signifi cantly decreased. The importance of ammonia in the bleaching of melanin has been observed for the conventional oxidant systems and has been reported previously in the literature (13). It is proposed that ammonia plays the same role in the lightening mechanism for both systems. Figure 6 shows the lightening for both oxidant systems with and without ammonia. For the conventional systems 4.5% hydrogen perox- ide was used with either 1.3% ammonia or sodium hydroxide adjusted to pH 10. The new oxidant was 4.5% hydrogen peroxide, 1.8% glycine and either 4.0% ammonium carbonate or 4.2% potassium hydrogen carbonate.
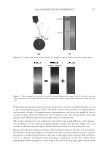
2008 TRI/PRINCETON CONFERENCE 213 (IV) ROLE OF GLYCINE The use of ammonium carbonate with hydrogen peroxide at pH 9 to lighten hair was fi rst reported in the patent literature in the 1960s (14) but it has only been commercialized in a very limited number of products. The issue found in early formulation work was that signifi cant fi ber damage occurred over multiple cycles. In particular for a system matched in lightening to a conventional oxidant system the tensile properties of the fi bers after 3 cycles of treatment were signifi cantly lower for the ammonium carbonate, hydrogen per- oxide system. It has been found that this loss of tensile strength can be prevented by the addition of glycine with no detrimental impact on the lightening. Figure 7 shows the tensile properties of the hair treated over multiple coloring cycles with the ammonium carbonate, hydrogen peroxide and glycine (pH 9) oxidant vs. the ammonium hydroxide, hydrogen peroxide (pH 10) oxidant. The systems were matched Figure 6. Role of ammonia in the lightening process. Figure 7. Tensile strength of oxidants over multiple coloring cycles.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)