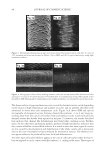
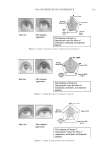
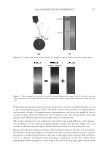
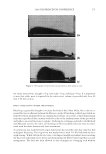
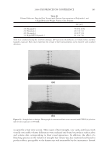
JOURNAL OF COSMETIC SCIENCE 214 in terms of lightening ability. The data demonstrate that the tensile strength of the new oxidant is at least equal to the conventional oxidant. The proposed mechanism for the action of glycine is its ability to control the concentra- tion of the carbonate radical inside the hair. The carbonate radical is a highly oxidizing species and if formed could be responsible for the observed tensile loss (15,16). The gly- cine may act as a radical scavenger by enabling fast electron transfer from the nitrogen of the glycine to the carbonate radical. The reaction scheme below is only hypothesized we have no direct evidence for the reactions, but it is supported by studies in the literature. (17,18). CONCLUSIONS The combination of ammonium carbonate, hydrogen peroxide and glycine at pH 9 pro- vides a lightening system that delivers equal to superior lightening to the conventional oxidant of ammonium hydroxide and hydrogen peroxide at pH 10. It is proposed that the lightening at this reduced pH is due to the introduction of a new oxidizing species, the peroxymonocarbonate ion, which is formed in-situ from the combination of hydrogen carbonate ions and hydrogen peroxide. This oxidant system has the advantage in that it has two levers, the hydrogen peroxide concentration and the ammonium carbonate con- centration, to change either the level of lightening or the time of lightening. Both can be changed to achieve higher lightening, lightening with lower hydrogen peroxide concen- trations or faster lightening. The introduction of glycine has allowed this system to de- liver this lightening without the previously observed negatives of tensile strength loss. It is proposed that the glycine acts as a scavenger of the carbonate radical. The formation of the carbonate radical is an undesired side reaction that is separate from the chemistry involved in the lightening of the melanin. Thus with the combination of the ammonium carbonate, hydrogen peroxide and glycine formulated at the optimum pH of 9.0 the lightening can be optimized without fi ber damage negatives. REFERENCES (1) J. J. Corbett, J. Soc. Cosmet. Chem., 24, 103 (1973). (2) F. E. Wall, in Cosmetics Science and Technology, E. Sagarin, Ed. (Interscience New York, 1957), Ch. 21. (3) J. Jachowicz, Hair damage and attempts to its repair, J. Soc. Cosmet. Chem., 38(4), 263–286 (1987).
2008 TRI/PRINCETON CONFERENCE 215 (4) D. P. Jones and W. P. Griffi th, J. Chem. Soc. Dalton Trans., 2526 (1980). (5) D. E. Richardson, H. Yao, K. M. Frank, and D. A. Bennett, J. Am. Chem. Soc., 122, 1729 (2000). (6) H. Yao and D. E. Richardson, J. Am. Chem. Soc., 99, 359 (1995). (7) B. S. Lane and K. Burgess, J. Am. Chem. Soc., 123, 2933 (2001). (8) J. Flanagan, J. Chem. Soc. Chem. Comm., 20 (1986). (9) O. Brovkina and B. Chernyshov, Russ. J. Inorg. Chem., 34, 166 (1989). (10) N. Wen and M. H. Brooker, J. Phys. Chem., 99, 359 (1995). (11) R. Beynon and J. Eastby, in Buffer Solutions—The Basics (Taylor & Francis, 2003). (12) D. A. Bennett, H. Yao, and D. E. Richardson, Inorg. Chem., 40, 2996 (2001). (13) P. Padmaja, K. J. Dube, S. A. Madison, and J. Bartalone, J. Cosmet. Sci., 54(4), 395 (2003). (14) Patent FR 1592939, Precision Value (1968). (15) O. Augusto, M. G. Bonini, A. M. Amanso, E. Linares, C. C. X. Santos, and S. L. De Menezes, Free Rad. Biol. Med., 32(9), 841 (2002). (16) G. Wu, Y. Katsumara, Y. Muroya, M. Lin, and T. Morioka, J. Phys. Chem. A, 106, 2430 (2002). (17) A. Rauk and D. A. Armstrong, J. Am. Chem. Soc., 122, 4185 (2000). (18) D. D. M. Wayner, K. B. Clark, A. Rauk, and D. A. Armstrong, J. Am. Chem. Soc., 119, 8925 (1997).
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)