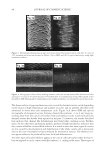
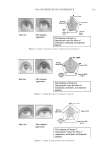
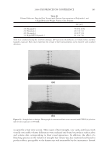
2008 TRI/PRINCETON CONFERENCE 223 OTHER PHYSICAL ANALYSIS Water contact angle. Contact angles of hair fi bers against water were measured using The Thermo Cahn Dynamic Contact Angle (DCA) Analyzer. The instrument uses Wilhelmy technology to measure surface properties of solid and liquid samples. Single fi ber contact angle technique was used where measurements were made with the fi ber advancing into water. The contact angle data was an average of over 20 fi ber measurements for each hair tress and meets the requirement of Cos θ 0.1 between the last two measurements. The hair diameter values required in contact angle measurement were measured by a laser micrometer instrument where the maximum diameter was employed. FTIR spectroscopic image analysis of hair fi bers. Fourier transform infrared imaging spectros- copy (FT-IRIS) is a novel infrared spectroscopic technique, which couples an FT-IR spec- trometer to an optical microscope with an array detector. The collection of data, combined with microscopic visualization of the sample, will essentially allow for “infrared imaging” of the sample. Thus, spatially resolved micron-resolution maps of the molecular compo- nents of sample can be obtained. Sample preparation. Hair bundle was emerged in a tissue freezing media liquid and quickly frozen in liquid nitrogen. The hair bundle was mounted with freezing medium on sample holder with cross section of hair fi bers facing out. The freezing hair bundle was sliced into a 4 micron thick of hair cross section using a micro slicing machine (Leica CM 1850) and was collected on CaF2 IR window for FT-IR imaging analysis use. The hair dye ingredients used for FTIR image analysis are listed in the above hair dye section. FTIR imaging. FT-IR images were acquired with Perkin Elmer Spotlight system, which consists of a linear array mercury-cadmium-telluride (MCT) detector along with an auto- mated high precision XY sample stage. A typical IR image could contain over 2000 spectra collected in minutes. Spectral Dimensions Isys 3.0 software was used for analysis and construction of the IR images. RESULTS AND DISCUSSIONS FACTORS AFFECTING HAIR COLOR FADING Effect of hair damage. Hair color loss is related to the quality of the hair. This is studied by soaking three types of dyed hair: bleached, grey and piedmont (natural white hair). Figure 1a shows the total color changes of the soaking liquors versus soaking time after soaking dyed bleached hair, dyed grey hair, and dyed piedmont hair in water. The effect of hair quality on color loss is further studied by conducting a multiple shampoo washing test with dyed bleached hair and dyed grey hair. Figure 1b shows the total color changes of dyed bleached hair and dyed grey hair after 10× shampoo washes by 12% SLES. The data demonstrates that dyed bleached hair lose dye more rapidly, about 40% faster than the dyed grey hair and dyed piedmont. This can be explained by the fact that the bleached hair is more damaged and has pores in increasing numbers and sizes, therefore, the dye molecules leach out from bleached hair much easily. Effect of surfactants. Effect of surfactant on hair dye dissolution in water was studied by soak- ing dyed bleached hair tresses in 1% aqueous surfactant solution. Three typical surfactants were selected in this study: anionic surfactant, sodium lauryl ether sulfate (SLES) cationic surfactant, cetrimonium chloride (CETAC) and coco trimonium chloride. Figure 2 shows
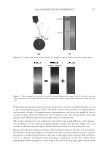
JOURNAL OF COSMETIC SCIENCE 224 the color change of hair dyed with two different colors after soaking in surfactant solutions. The data indicates that surfactants, especially cationic surfactants (CETAC and coco trimo- nium chloride), increase hair dye dissolution in water. The cationic surfactants evaluated increases hair dye dissolution much more than the anionic surfactant, SLES. Compared with water alone, CETAC increases hair dye dissolution by 127% for dark brown color after 1 hour of soaking, while the anionic surfactant, SLES, increases the hair dye dissolu- tion by only 47% (Figure 2b). Figure 2a shows that another cationic surfactant, coco tri- moinium chloride, increases hair dye dissolution signifi cantly more than SLES in deep red colored hair soaked over time at pH 5. This is explained by the fact that the surfactants increase hair dye dissolution by increasing wetting properties on the hair fi ber surface, which facilitates moisture going inside the hair shaft and removes the hair dye. Effect of pH of soaking liquor. The effect of soaking liquor pH on hair dye dissolution was studied by soaking dyed bleached hair tresses in three buffered aqueous solutions. pH 3 Figure 1. Hair dye dissolution in water: effect of hair damage. (a) Dyed hair soaked in water, intense red color. (b) 10× shampoo washes with 12% SLES. Figure 2. Effect of surfactants on hair dye dissolution in water. (a) Deep red colored bleached hair, pH 5. (b) Dark brown colored bleached hair, 1-hour soaking, pH 7.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)