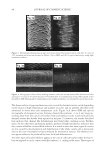
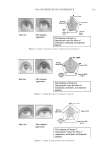
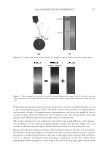
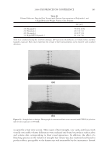
JOURNAL OF COSMETIC SCIENCE 210 lightening for this new oxidant at pH 10 is being driven by the presence of the perhy- droxyl anion. One reason why the concentration of the peroxymonocarbonate ion de- creases at pH 10 is its deprotonation to the peroxymonocarbonate dianion (12) as shown in Equation 10. (10) (II) LIGHTENING AS A FUNCTION OF HYDROGEN PEROXIDE AND CARBONATE CONCENTRATION A series of cream formulations were made at the optimal pH for the new oxidant system (pH 9.0–9.3). In the fi rst set of formulations the ammonium carbonate concentration was varied from 2% to 5% with a fi xed hydrogen peroxide concentration of 4.5% and glycine concentration of 1.8%. A second series of cream formulations were made with a hydrogen peroxide concentration that was varied from 3% to 9% and a fi xed glycine (1.8%) and ammonium carbonate concentration (2%). All of these products were then applied to medium brown untreated human hair samples for a period of 30 minutes in a temperature controlled environment of 30°C. Figure 4 shows the lightening data as a function of the carbonate concentration and Figure 5 shows the lightening data as a func- tion of hydrogen peroxide concentration. These data again clearly demonstrate that this combination of ammonium carbonate, hydrogen peroxide and glycine at pH 9 can give effective lightening. The lightening values are similar to these obtained for a conven- tional oxidant system of ammonium hydroxide and hydrogen peroxide at pH 10. It also shows that the lightening can be increased either by changing the carbonate concentra- tion or the hydrogen peroxide concentration. As the carbonate concentration and the hydrogen peroxide concentration are increased, lightening also increases. The reason for this lightening dependence on the hydrogen peroxide and carbonate con- centrations can be explained by consideration of the equilibrium responsible for forming the peroxymonocarbonate ion (Equation 1). As either the hydrogen peroxide or ammo- nium carbonate concentrations are increased the equilibrium will be shifted to the right and form more of the peroxymonocarbonate ion. This relationship has been confi rmed with the 13 C NMR studies where the concentration of the peroxymonocarbonate ion was measured as a function of carbonate and hydrogen peroxide concentrations (see Table II). Thus with this new oxidant we now have two ‘levers’ that can increase the lightening. This is different from the conventional ammonium hydroxide/hydrogen peroxide/pH 10 oxidant where the only lever to increase the concentration of the oxidant species is in- creasing the hydrogen peroxide concentration. Table I pH Dependence of the Peroxymonocarbonate Ion and Perhydroxyl Anion Concentrations pH Peroxymonocarbonate ion (mol−l) Perhydroxyl anion (mol−1) 8.5 0.031 0.000 9.0 0.021 0.002 10.0 0.000 0.020
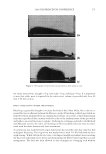
2008 TRI/PRINCETON CONFERENCE 211 Figure 4. Lightening as a function of carbonate concentration. Figure 5. Lightening as a function of hydrogen peroxide concentration. Table II Concentration of Peroxymonocarbonate Ion as a Function of Ammonium Carbonate and Hydrogen Peroxide Concentration % Ammonium carbonate % Hydrogen peroxide Peroxymonocarbonate ion (mM) 1.5 3.1 22.3 1.5 6.3 45.9 3.5 3.0 32.7 3.5 6.0 64.9 5.1 3.0 52.3 5.0 6.0 74.0 One benefi t of this lightening system is its fl exibility, i.e. the carbonate and hydrogen peroxide concentrations can be adjusted as two independent levers to alter the lightening. This can be used to match the current lightening performance of a conventional oxidant
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)