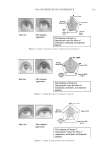
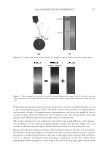
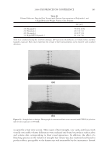
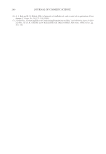
JOURNAL OF COSMETIC SCIENCE 208 for the new oxidant system the lightening at pH 9 is very close to what is achieved at pH 10. These two charts also show that the new oxidant at pH 9 can achieve similar lighten- ing to the conventional oxidant at pH 10 (~8–12 dL). These results imply that a different oxidant is being formed with the addition of the am- monium carbonate. It is proposed that this new oxidant is the peroxymonocarbonate ion which is formed in-situ from the combination of hydrogen peroxide and hydrogen carbon- ate ions (see Equation 1). The formation of this species is documented in the literature (4,5), and the species has been shown to be an effective oxidant (6,7). (1) To study the formation of this species at the concentrations used in the oxidant system tested for lightening the hair we used 13 C NMR (8,9). This technique also allows us to track the formation of this species as a function of pH. Figure 3 shows a typical 13 C NMR spectrum that was obtained for a solution containing ammonium carbonate, hydrogen peroxide and glycine at pH 9. The peroxymonocarbon- ate ion can be monitored and its relative concentration measured with these spectra. The spectrum clearly shows that the peroxymonocarbonate ion is formed at the concentrations used in the lightening experiment. The above 13 C NMR spectrum was acquired from a solution of 4.5% w/w ammonium carbonate/0.5% w/w 99% 13 C atom enriched sodium carbonate, 2% w/w glycine and 6% H2O2. The spectrum also shows different species that are formed in the equilibria between the ammonia, the carbonate ions, the hydrogen carbonate ions, the hydrogen peroxide and the glycine (10). Equations 2–9 show these equilibria in more detail. Figure 2. Lightening of oxidant systems at pH 9 and 10.
2008 TRI/PRINCETON CONFERENCE 209 NH4+ + H2O ↔ NH3 + H3O+ pKa = 9.3 (2) HCO3− + H2O ↔ CO32− + H3O+ pKa = 10.3 (3) CO32− + NH4+ ↔ HCO3− + NH3 (4) HCO3− + NH3 ↔ H2NCOO− + H2O (5) H2NCOO− + H2O ↔ CO32− + NH4+ (6) + H3NCH2COO− + H2O ↔ H2NCH2COO− + H3O+ pKa = 9.7 (7) H2NCH2COO− + HCO3− ↔ − OOCNH2CH2COO− + H2O (8) H2O2 ↔ HOO− + H+ pKa = 11.6 (9) The 13 C NMR spectra can explain the pH dependence observed for the two oxidant sys- tems. For the conventional oxidants the lightening species formed is the perhydroxyl anion (HOO−) which is formed as the hydrogen peroxide is deprotonated at higher pHs (10). For the new oxidant system there are two possible oxidants that are formed, the peroxymonocarbonate ion and the perhydroxyl anion. Table I below shows the concentra- tion of these two species as a function of pH. The concentrations used were 4.0% ammo- nium carbonate, 4.5% hydrogen peroxide, 1.8% glycine for the new oxidant and 1.3% ammonia, 4.5% hydrogen peroxide for the conventional oxidant. The concentration of the peroxymonocarbonate ion is measured from the 13 C NMR spectra and the concentra- tion of the perhydroxyl anion is calculated from the pKa using the Henderson-Haselbach equation (11). These data demonstrate that the peroxymonocarbonate ion is present at pH 8.5 and 9 and it is proposed that this is the species that is responsible for the pH 9 lightening of the new oxidant. At pH 10 the peroxymonocarbonate ion is not present but it is proposed that the Figure 3. NMR of ammonium carbonate, hydrogen peroxide, and glycine solution at pH 9.0.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)