JOURNAL OF COSMETIC SCIENCE 206 achieving good gray coverage without the negative of the shade going too dark. In this case, the oxidant lightens the underlying pigment and previously deposited color thus allowing for the addition of dye to achieve good gray coverage with no decrease in the darkness of the shade and no build-up of dye and darkening over multiple usage occa- sions. The lighting is also important for the achievement of vibrant shades, for example the red shades. However, the oxidant is also responsible for the fi ber changes that can occur as the hair is colored for multiple treatments leading to consumer negatives such as poor shine, poor feel and reduced strength (3). The search for an alternative oxidant that can lighten hair but also have no negatives on fi ber damage has long been one of interest to companies that develop hair coloring products. In this paper we explore the alternative oxidant of am- monium carbonate, hydrogen peroxide and glycine buffered at pH 9.0 and we describe its proposed mechanism of action. This oxidant participates in the same color forming reactions as conventional oxidants and it can be used to match the typical range of shades. However, this color formation chemistry will not be discussed as part of this paper. EXPERIMENTAL Caucasian untreated mixed hair (medium brown), obtained from a commercial source (IHIP, New York), was formed into swatches (16 cm, 1.5 g) and used for all the testing. A range of cream formulation products were made to test the lightening effi ciency of the new oxidant as a function of pH, ammonium carbonate concentration and hydrogen per- oxide concentration. A concentrated cream base was made using 5.0% of Crodafos CES® (a mix of cetearyl alcohol, dicetylphosphate and ceteth-10 phosphate) which was dis- solved in hot DI water (85°C), neutralized with sodium hydroxide and then allowed to slowly cool. The ammonium carbonate, glycine and hydrogen peroxide were added to the remaining water and then mixed into the concentrated cream base until it was homoge- nous. The pH was adjusted with sodium hydroxide. The same concentrated cream base was used to make the formulations with the conven- tional oxidant. In this system the pH was adjusted with acetic acid. The fi nal cream formulation (4 g of product per g of hair) was thoroughly applied to the hair and then kept for 30 minutes at a controlled temperature of 30°C. The color of the hair was measured with a bench top Minolta CM3600D spectrophoto m- eter. Lab values were calculated under D65 illuminant, 10° observer, specular included. The lightening (dL) was calculated as the difference in L value between the fi nal color and the starting color on the untreated hair. 13 C NMR spectra were acquired on a Bruker Avance spectrometer operating at a 13 C fre- quency of 100.6 MHz. All spectra were acquired with an inverse gated 13 C experiment with a relaxation delay of 30 s, SW of 249 ppm and FID acquisition time of 1.3 s and 16 scans of spectral averaging were used. In the data presented later, in order to boost sensi- tivity, 10% of the quoted mass of ammonium carbonate solutions was replaced by 99% 13 C atom percent enriched sodium carbonate (Sigma Aldrich).
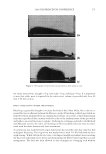
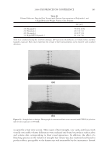
2008 TRI/PRINCETON CONFERENCE 207 The tensile properties of the fi bers were measured using a Diastron Miniature tensile tester (MTT 675) equipped with laser micrometer in a water saturated environment. At least 100 fi bers were run for each measurement point. RESULTS A series of cream formulations were made containing ammonium carbonate, hydrogen peroxide and glycine and compared to a conventional oxidant system of ammonium hy- droxide and hydrogen peroxide at pH 10. This is the oxidant system used in the majority of retail and professional hair colorant products. The lightening of this new oxidant was investigated as a function of several important parameters: (i) Lightening as a function of pH (ii) Lightening as a function of hydrogen peroxide and carbonate concentration (iii) Lightening as a function of source of carbonate (i.e. ammonium carbonate vs. ammonium carbamate vs. potassium hydrogen carbonate) (I) LIGHTENING AS A FUNCTION OF pH A series of cream formulations were made with ammonium carbonate (4%), glycine (1.8%) and hydrogen peroxide (4.5%) at a pH range of 8.5 to 10.5. These cream products were applied to medium brown untreated human hair samples for a period of 30 minutes in a temperature controlled environment of 30°C. The lightening of the hair after these treat- ments was measured and Figure 1 demonstrates the pH dependence of this lightening. The fi gure shows that the optimal pH is between pH 9 and 9.5 but signifi cant lightening is still achieved at pH 8.7. This pH profi le is very different from the profi le found for the conventional oxidant system where the lightening maximum is found at approximately pH 10 and the lightening decreases sharply below pH 10. Figure 2 shows the lightening for the two oxidant systems at pH 9 and pH 10. For the con- ventional oxidant system the cream formulations were made at a hydrogen peroxide concen- tration of 4.5% active with the pH adjusted with an ammonium hydroxide/acetic acid buffer (1.3% ammonia) to pH 9 and 10. The fi gure clearly shows that for the conventional oxidant the lightening at pH 9 is approximately 60% of the lightening at pH 10 but that Figure 1. Ammonium carbonate + peroxide + glycine as a function of pH.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)