338 JOURNAL OF COSMETIC SCIENCE mM Tris) (3). In this study, we designated this soluble protein fraction as "labile protein." Eluted proteins are released from hair, whereas labile proteins remain in hair upon chemical treatments such as permanent waving or bleaching. The electrophoretic profile of labile proteins was substantially similar to that of proteins eluted by perma- nent waving or bleaching (4). Both labile and eluted fractions were mainly constituted of low molecular proteins, and their major component was identified to be ubiquitin (4). Labile proteins might reflect the cumulative effect of chemical treatments on hair fiber. We hypothesized that the labile protein levels of damaged hair correlate with the denaturation of hair protein by permanent waving or bleaching. Through the quanti- tative analyses of the labile protein, we found that labile protein levels were dramatically increased upon permanent waving and bleaching treatments. Here we propose a new mechanism of hair damage that contains formation of labile protein. Furthermore, the labile protein level could be used for a superior index that enables sensitive, accurate, and reproducible assessments of hair damage. MATERIALS AND METHODS MATERIALS Two types of hair fiber samples were collected from Japanese individuals. One was a short hair sample, a mixture of 2-cm-long hair fibers, on average derived from men with short hair. The other was a long hair sample, a hair strand 30-cm-long derived from a Japanese woman. These hair fibers had not been exposed to chemical treatments such as permanent waving or bleaching. PERMANENT WAVING AND BLEACHING All treatments were done at 37øC with a liquid-to-fiber ratio of 50:1. Typically, 10 g of the hair samples were treated. Bleach treatments were done for one hour or 16 hours with a bleach solution containing 6% hydrogen peroxide and 0.5% ammonia. Perm treatments were carried out using a waving agent containing 6% ammonium thiogly- colate, 1% 2-aminoethanol, and 0.45% ammonia for 15 minutes or 60 minutes. After rinsing with deionized water, the waved hair was neutralized by 7 % sodium bromate for 15 minutes. After exhaustive rinsing following all treatments, the treated hair was dried at room temperature. EXTRACTION OF LABILE PROTEINS IN HAIR All extractions were done at 37øC for 16 hours with a liquid-to-fiber ratio of 50:1. In the case of the long hair sample, samples were cut to approximately 2.5 cm. Typically, the hair samples (100 mg or 0.6 g) were extracted in 5 ml or 30 ml of 200 mM Tris containing 200 mM 2-mercaptoethanol. The eluted protein under this reducing condi- tion was defined as "labile protein."
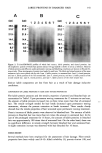
LABILE PROTEINS IN DAMAGED HAIR 339 PREPARATION OF PROTEIN SAMPLES All protein samples were first passed through a cellulose acetate filter (0.8 lam, Dismic 25CS, Toyo Roshi Ltd, Tokyo, Japan). A small sample concentration, typically 3.5 ml of protein extract, was carried out using a centrifugal filter device (Centricon YM-3, Millipore, MA). The concentrates were subjected to protein assays. Whole hair protein extract was prepared using a solution containing dithiothreitol and sodium dodecylsul- fate as previously described (3). In the case of samples requiring further condensation, the extract was concentrated using a stirred cell system (Amicon 8050, Millipore, MA) equipped with an ultra-filtration membrane (YM-3, Millipore, MA). The resultant concentrate was further condensed using the centrifugal filter device. When the proteins eluted into the permanent waving or bleaching lotions were concentrated, the solvents of resultant concentrates were diluted once by distilled water. Following reconcentration, the concentrates were then diluted by 200 mM Tris containing 200 mM 2-mercaptoethanol. Final concentration was carried out using both a stirred cell and the centrifugal filter device. PROTEIN ASSAY Protein contents were measured by a dye-binding assay (BioRad protein assay, BioRad, CA) using bovine albumin as a standard. TENSILE PROPERTIES For the measurement of tensile properties, the hair tress (1.5 g) made from the long hair sample was permed or bleached. Tensile properties of single hair fibers were determined at 25øC and 60% relative humidity using a tensile tester (Dianos A2, Toto, Tokyo). ELECTROPHORETIC ANALYSIS The electrophoretic analyses were performed using a size separation method employing tricine/sodium dodecyl sulfate/polyacrylamide gel (16.5%T, 6%C) electrophoresis under the same conditions used by Schiigger and von Jagow (5). Size-separated proteins were electroblotted onto a polyvinylidene difluoride membrane (Immobilon Psq, Millipore, CA). Protein blots were stained with Coomassie Brilliant Blue R (Sigma, MO). RESULTS COMPARISON OF LABILE PROTEINS IN HAIR AND ELUTED PROTEINS UNDER PERMANENT WAVING AND BLEACHING TREATMENTS Short hair samples were treated with permanent waving or bleaching lotions, and then subjected to analyses of both labile and eluted proteins. The changes of labile proteins and eluted proteins by permanent waving are shown in Figure 1A. The amount of labile proteins was below 0.3 mg per one gram of untreated hair. This value was smaller than the amount of proteins eluted during the first treatment of permanent waving. The amount of lablie proteins in the hair upon the first permanent waving treatment was
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)