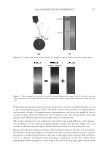
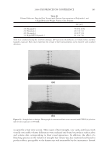
2008 TRI/PRINCETON CONFERENCE 209 NH4+ + H2O ↔ NH3 + H3O+ pKa = 9.3 (2) HCO3− + H2O ↔ CO32− + H3O+ pKa = 10.3 (3) CO32− + NH4+ ↔ HCO3− + NH3 (4) HCO3− + NH3 ↔ H2NCOO− + H2O (5) H2NCOO− + H2O ↔ CO32− + NH4+ (6) + H3NCH2COO− + H2O ↔ H2NCH2COO− + H3O+ pKa = 9.7 (7) H2NCH2COO− + HCO3− ↔ − OOCNH2CH2COO− + H2O (8) H2O2 ↔ HOO− + H+ pKa = 11.6 (9) The 13 C NMR spectra can explain the pH dependence observed for the two oxidant sys- tems. For the conventional oxidants the lightening species formed is the perhydroxyl anion (HOO−) which is formed as the hydrogen peroxide is deprotonated at higher pHs (10). For the new oxidant system there are two possible oxidants that are formed, the peroxymonocarbonate ion and the perhydroxyl anion. Table I below shows the concentra- tion of these two species as a function of pH. The concentrations used were 4.0% ammo- nium carbonate, 4.5% hydrogen peroxide, 1.8% glycine for the new oxidant and 1.3% ammonia, 4.5% hydrogen peroxide for the conventional oxidant. The concentration of the peroxymonocarbonate ion is measured from the 13 C NMR spectra and the concentra- tion of the perhydroxyl anion is calculated from the pKa using the Henderson-Haselbach equation (11). These data demonstrate that the peroxymonocarbonate ion is present at pH 8.5 and 9 and it is proposed that this is the species that is responsible for the pH 9 lightening of the new oxidant. At pH 10 the peroxymonocarbonate ion is not present but it is proposed that the Figure 3. NMR of ammonium carbonate, hydrogen peroxide, and glycine solution at pH 9.0.
JOURNAL OF COSMETIC SCIENCE 210 lightening for this new oxidant at pH 10 is being driven by the presence of the perhy- droxyl anion. One reason why the concentration of the peroxymonocarbonate ion de- creases at pH 10 is its deprotonation to the peroxymonocarbonate dianion (12) as shown in Equation 10. (10) (II) LIGHTENING AS A FUNCTION OF HYDROGEN PEROXIDE AND CARBONATE CONCENTRATION A series of cream formulations were made at the optimal pH for the new oxidant system (pH 9.0–9.3). In the fi rst set of formulations the ammonium carbonate concentration was varied from 2% to 5% with a fi xed hydrogen peroxide concentration of 4.5% and glycine concentration of 1.8%. A second series of cream formulations were made with a hydrogen peroxide concentration that was varied from 3% to 9% and a fi xed glycine (1.8%) and ammonium carbonate concentration (2%). All of these products were then applied to medium brown untreated human hair samples for a period of 30 minutes in a temperature controlled environment of 30°C. Figure 4 shows the lightening data as a function of the carbonate concentration and Figure 5 shows the lightening data as a func- tion of hydrogen peroxide concentration. These data again clearly demonstrate that this combination of ammonium carbonate, hydrogen peroxide and glycine at pH 9 can give effective lightening. The lightening values are similar to these obtained for a conven- tional oxidant system of ammonium hydroxide and hydrogen peroxide at pH 10. It also shows that the lightening can be increased either by changing the carbonate concentra- tion or the hydrogen peroxide concentration. As the carbonate concentration and the hydrogen peroxide concentration are increased, lightening also increases. The reason for this lightening dependence on the hydrogen peroxide and carbonate con- centrations can be explained by consideration of the equilibrium responsible for forming the peroxymonocarbonate ion (Equation 1). As either the hydrogen peroxide or ammo- nium carbonate concentrations are increased the equilibrium will be shifted to the right and form more of the peroxymonocarbonate ion. This relationship has been confi rmed with the 13 C NMR studies where the concentration of the peroxymonocarbonate ion was measured as a function of carbonate and hydrogen peroxide concentrations (see Table II). Thus with this new oxidant we now have two ‘levers’ that can increase the lightening. This is different from the conventional ammonium hydroxide/hydrogen peroxide/pH 10 oxidant where the only lever to increase the concentration of the oxidant species is in- creasing the hydrogen peroxide concentration. Table I pH Dependence of the Peroxymonocarbonate Ion and Perhydroxyl Anion Concentrations pH Peroxymonocarbonate ion (mol−l) Perhydroxyl anion (mol−1) 8.5 0.031 0.000 9.0 0.021 0.002 10.0 0.000 0.020
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)