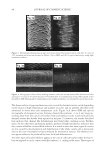
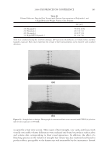
2008 TRI/PRINCETON CONFERENCE 207 The tensile properties of the fi bers were measured using a Diastron Miniature tensile tester (MTT 675) equipped with laser micrometer in a water saturated environment. At least 100 fi bers were run for each measurement point. RESULTS A series of cream formulations were made containing ammonium carbonate, hydrogen peroxide and glycine and compared to a conventional oxidant system of ammonium hy- droxide and hydrogen peroxide at pH 10. This is the oxidant system used in the majority of retail and professional hair colorant products. The lightening of this new oxidant was investigated as a function of several important parameters: (i) Lightening as a function of pH (ii) Lightening as a function of hydrogen peroxide and carbonate concentration (iii) Lightening as a function of source of carbonate (i.e. ammonium carbonate vs. ammonium carbamate vs. potassium hydrogen carbonate) (I) LIGHTENING AS A FUNCTION OF pH A series of cream formulations were made with ammonium carbonate (4%), glycine (1.8%) and hydrogen peroxide (4.5%) at a pH range of 8.5 to 10.5. These cream products were applied to medium brown untreated human hair samples for a period of 30 minutes in a temperature controlled environment of 30°C. The lightening of the hair after these treat- ments was measured and Figure 1 demonstrates the pH dependence of this lightening. The fi gure shows that the optimal pH is between pH 9 and 9.5 but signifi cant lightening is still achieved at pH 8.7. This pH profi le is very different from the profi le found for the conventional oxidant system where the lightening maximum is found at approximately pH 10 and the lightening decreases sharply below pH 10. Figure 2 shows the lightening for the two oxidant systems at pH 9 and pH 10. For the con- ventional oxidant system the cream formulations were made at a hydrogen peroxide concen- tration of 4.5% active with the pH adjusted with an ammonium hydroxide/acetic acid buffer (1.3% ammonia) to pH 9 and 10. The fi gure clearly shows that for the conventional oxidant the lightening at pH 9 is approximately 60% of the lightening at pH 10 but that Figure 1. Ammonium carbonate + peroxide + glycine as a function of pH.
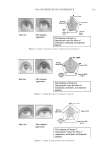
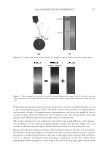
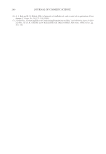
JOURNAL OF COSMETIC SCIENCE 208 for the new oxidant system the lightening at pH 9 is very close to what is achieved at pH 10. These two charts also show that the new oxidant at pH 9 can achieve similar lighten- ing to the conventional oxidant at pH 10 (~8–12 dL). These results imply that a different oxidant is being formed with the addition of the am- monium carbonate. It is proposed that this new oxidant is the peroxymonocarbonate ion which is formed in-situ from the combination of hydrogen peroxide and hydrogen carbon- ate ions (see Equation 1). The formation of this species is documented in the literature (4,5), and the species has been shown to be an effective oxidant (6,7). (1) To study the formation of this species at the concentrations used in the oxidant system tested for lightening the hair we used 13 C NMR (8,9). This technique also allows us to track the formation of this species as a function of pH. Figure 3 shows a typical 13 C NMR spectrum that was obtained for a solution containing ammonium carbonate, hydrogen peroxide and glycine at pH 9. The peroxymonocarbon- ate ion can be monitored and its relative concentration measured with these spectra. The spectrum clearly shows that the peroxymonocarbonate ion is formed at the concentrations used in the lightening experiment. The above 13 C NMR spectrum was acquired from a solution of 4.5% w/w ammonium carbonate/0.5% w/w 99% 13 C atom enriched sodium carbonate, 2% w/w glycine and 6% H2O2. The spectrum also shows different species that are formed in the equilibria between the ammonia, the carbonate ions, the hydrogen carbonate ions, the hydrogen peroxide and the glycine (10). Equations 2–9 show these equilibria in more detail. Figure 2. Lightening of oxidant systems at pH 9 and 10.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)