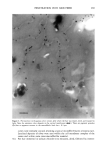
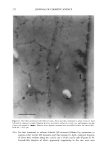
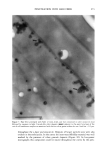
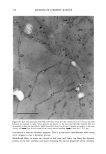
j. Cosmet. Sci., 52, 255-263 (September/October 2001) Study of silicone vehicles for Aloe vera V. GALLARDO, R. MONTOYA, and M. A. RUIZ, Pharmacy and Pharmaceatical Technology Department, School of Pharmacy, University of Granada, E-18071 Granada, Spain. Accepted for pablication May 31, 2001. Synopsis The effect of different surfactants on the synthesis of a silicone latex is studied. Distinct formulations were mixed and then tested with regard to stability as a function of time and temperature. The ones with the best characteristics for acting as a vehicle for the active cosmetic ingredient Aloe vera are indicated. INTRODUCTION The systems studied are dispersions of a silicone polymer in water to obtain the so-called latexes (1,2), which are among the newest forms to administer drugs and cosmetic agents (3-5). In recent years a great variety of latexes have been obtained, with different polymers and varying particle and/or droplet size, depending on the preparation con- ditions. Many latexes have been synthesized using acrylic polymers and cellulose de- rivatives (6), but very few with silicones. Given the wide field of uses open to formulations based on latex and particularly on silicone latex, the aim of this work was to obtain a stable pseudolatex formula for incorporation in different active compounds both during and after the preparation phase. The active compound chosen for incorporation in the latex is Aloe vera, the genus Aloe comprising some 150 species. Aloe vera is adapted to dry tropical climates, because of which the plants are rich in polysaccharides that aid in water retention. Aloe vera is currently used in pharmaceutics as a cicatrizant agent (7,8) and as a vehicle for corticoids. It is very commonly utilized in cosmetics for its hydrant and sunscreen properties (9). EXPERIMENTAL MATERIALS ß Aloe vera gel (1:1) from Aloe Corp. Supplied by Zeus Quimica, Barcelona, Spain. ß Silicone oil (Dow Corning 245). Dimethyl cyclic pentamer. Obtained from Dow Corning Ltd. (Brussels, Belgium) 255
256 JOURNAL OF COSMETIC SCIENCE Abil EM 90, W/O an emulsifier. Cetyl dimethicone copolyol. Supplied by Th Gold- schmidt Ag (Essen, Germany) Abil WE 09, a W/O emulsifier. Cetyl dimethicone copolyol (and) polyglycerol-4- iso-stearate (and) hexyl laurate. Supplied by Th Goldschmidt Ag (Essen, Germany) Abil B8845, polysiloxane polyether copolymers, a silicone surfactant with a watery external phase (O/W). Supplied by Th Goldschmidt Ag (Essen, Germany) LSNa, a surfactant with a watery external phase (O/W) n-decane Distilled water METHOD Several formulations were elaborated based on the formula of Vanderhoff et al. (10) and modifying the surfactant (Table I). The following formulations were chosen: LSNa (O/W anionic surfactant), Abil 8851 (W/O silicone surfactant), Abil EM 90 (W/O silicone surfactant), and Abil WE 09 (W/O silicone surfactant) at different concentrations. We obtained formulations that were studied for both organoleptic properties and sta- bility, verifying that the formulas with Abil EM 90 and Abil WE 09 provided the best characteristics, and in these formulations we studied the influence of the concentration of emulsifier (Table II). Test release. Ointments containing Aloe vera were placed in a 6-cm petri dish with lightly acidic water and shaken at 60 rpm at 37øC in a thermosrated bath. This shaking speed was shown to be sufficient to mix the Aloe vera throughout the aqueous phase after its release from the vehicle, without leading to significant dissolution of the base. The amount of Aloe vera released was determined after 0.5, 1, 2, 4, 6, 8, and 24 h by measuring absorption at 256 nm with a Perkin-Elmer running lambda 2 mod.127 spectrophotometer. Blanks containing no active principle were prepared in the same way as samples, and tested to compensate for possible interference between the components of the formulation. At least three determinations were done for each data point. RESULTS AND DISCUSSION The pH was measured with a direct method using a Crison mcropH 2001 meter (Alella, Barcelona, Spain) (11). In addition, we used phase separation with n-butanol and tri- Table I Formulations Modifying the Surfactant Formulation 1 Formulation 2 Formulation 3 Formulation 4 DC 245 24.6% 24.6% 24.6% n-decane 0.37% 0.37% 0.37% H20 73.98% 73.98% 73.98% LSNa + 1% -- -- Abil B 8851 -- 1% -- Abil EM 90 -- -- 1% Abil WE 09 -- -- 24.6% O.37% 73.98%
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)