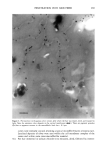
2001 ANNUAL SCIENTIFIC SEMINAR 339 DYE LOCALIZATION AND ESTIMATION OF LIPID PATHLENGTH IN HUMAN STRATUM CORNEUM Gerald B. Kasting', Ph.D., P.S. Talreja', N. K. Kleene:, W. L. Piekens 3 and T. F. Wang • 1 University of Cincinnati, College of qharmacy, 2Dept of Cell Biology, Neurobiology and Anatomy, 3Skin Sciences Institute, Children s Hospital Research Center University of Cincinnati and 4Dept of Chemical Engineering, State University of New York at Buffalo Introduction. Since the elucidation of the biphasic structure of the stratum corneum (SC), i.e., flattened, proteinaceous corneocytes embedded within an ordered !ipid matrix, much has been written about the implications of this structure for solute transport. The barrier properties in skin reside primarily within the SC and are determined by the permeabilities of both corneocyte and !ipid phases. In recent years, many workers have argued that the primary transport pathway for most materials traversing the SC is intercellular [1-4]. If this is true, it follows that the arrangement of the corneocytes within the lipid matrix is a key determinant to the skin's permeability, as it would influence the effective pathlength for diffusion. Methods have been developed to calculate the diffusion of permeants traversing orderly arrays of corneocytes via intercellular [4, 5] and combined interce!!ular/transceilular [6, 7] pathways. Calculations to date have been based on two-dimensional (2-D) representations of the SC. Modern confocal microscopy techniques provide a tool for evaluating one key property - !ipid pathway tortuosity - in more detail than previously possible, by allowing direct observation of the 3-D structure. Furthermore, confocal techniques enable improved dye localization in tissue, by eliminating out-of-focus flare. We have studied corneocyte arrangement and dye localization in alkali-expanded human SC using both 2-D and 3-D fluorescence microscopy [g]. Lipid pathlengths and geometrical tortuosity factors, x•, in expanded SC were determined by analysis of the 2-D images. These values were then corrected for the effects of swelling to arrive at corresponding values x z for the membrane prior to expansion. Model calculations were conducted to !ink these 2-D values with actual 3-D tortuosities. Finally, microscopic images obtained from human SC stained with dansyl chloride, then expanded, were analyzed to determine the location of the dye within the tissue. Methods. Cross-sections of alkali-expanded human SC [9] were optically examined by a combination of brightfield microscopy, differential interference contrast (DIC), and fluorescence microscopy techniques. Pathlengths through the intercellular lipiris were determined manually and divided by the local SC thickness to estimate x• for expanded SC. The tortuosity prior to expansion, x v was calculated fi'om Eq. I: oe, rg -1 = T,(% -l) (1) where Et and Et were the estimated lateral and transverse swelling ratios in the tissue [g]. The relationship of these values with 3-D tortuosities was established by methods described elsewhere [10]. Selected SC samples were stained with Nile red and examined in three dimensions using a laser scanning confocal microscope, using excitation at 488 rim. Results. A representative image of human SC is shown in Fig. 1. The corneocyte arrangement shows partial columnar alignment, but less than that assumed in some "brick-and-mortar" structural models for the SC [4, 5]. Similar arrangements were seen for the other samples analyzed. The results of !ipid pathlength analyses for these samples are summarized in Table I. The average tortuosity factor in the expanded state was x• = 3.7 _+ 0.7. This led to a calculated value prior to expansion of x z = 12.7 _+ 2.3. According to our current estimates, this 2-D value for tortuosity will translate into a value of about 10 in three dimensions (Fig. 2). Two-dimensional brick-and-mortar models [4, 5] yield values for x z ranging fi'om 5.8 to 22.5 [8]. ..... Figure 1. Light mi•o•aph of aikali-expan&d human SC stained wi• methy!•e chloride. •is •mple yielded a value of 3.3 for z• and 13.2 for
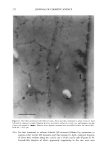
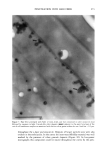
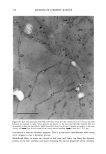
340 JOURNAL OF COSMETIC SCIENCE Table 1. Geometrical tortuosity of lipid pathway in expanded (xs•) and unexpanded (x•) human SC. Donor, sex, age, site Mean + SD • IOO• 5.9 13.2 3.6 !0.7 5.9 16.4 6.1 12.0 3.3 11.1 # 1, unspecified 3.3 + 0.1 (4) #2, male, 72, back 4.0 + 0.7 (4) #1, unspecified 3.9 + 0.6 (4) #2, male, 72, back 3.0 + 0.7 (5) #2, male, 72, back 4.4 + 0.7 (5) Mean + SD 3.7 + 0.7 12.7ñ2.3 Transverse expansion factor estimated as described in Ref. [8] Calculated according to Eq. ! using Et = !.11 [1 I]. o.oo 4 oo e oo 12.•1•1 1 a.•o 20 •o 2-0 TF Figure 2. Relationship of 2-D and 3-D tortuosity factors. Figure 3 shows a 2-D fluorescence image of human SC stained with dansyl chloride, then expanded. Dansyl chloride is a reactive dye that binds covalently to free amino groups. In skin, it primarily labels keratins and other proteins. The presence of fluorescence within the corneocytes suggests their permeability to the dye in their native, unexpanded state. We are in the process of confirming this result using confocal techniques to minimize the possibility of artifact in these measurements. tntensl•y .=..• Discussion. These experiments form part of a broader program in our laboratories to re-examine the mechanism of percutaneous absorption using a microtransport approach. The questions of penetration pathways through the SC and the relative permeabilities of corneocyte and lipid phases to permeants of different size and lipophilicity have not been satisfactorily resolved. Better answers to these questions will form the basis for improved predictive models for topical/transdermal drug delivery and dermal exposure and risk assessments. 10 U• • Conclusions. Fluorescence, brightfield, and DIC microscopy, in combination with alkaline expansion and a geometrical model to account for the effect of swelling, can be used to estimate intercellular lipid pathlengths across human SC. Confocal optics show potential for extending this work from two to three dimensions. Application of these techniques to a small number of human SC samples led to calculated average lipid pathlengths, relative to the SC width, of about 3.7 after expansion and 12.7 in unexpanded membranes. Figure 3. Fluorescence image of human SC stained with dansyl chloride. Acknowledgement. Financial support was provided by the Procter & Gamble Company's International Program for Animal Alternatives and by the National Science Foundation GOALI program. I. Flynn, G.L., in: Percutaneous Absorption (R.L. Bronaugh and H.I. Maibach, eds.), pp.27-5 I. Marcel Dekker, New York (1985). 2. Bodde, H.E., van den Brink, I., Koerten, H.K., and de Haan, F.H.N., J. Contr. Rel. 15:227-236 (1991). 3. Potts, R.O. and Francoeur, M., J. Invest. Dermatol. 96:495-499 (1991). 4. Johnson, M.E., Blankschtein, D., and Langer, R., J. Pharm. Sci. 86:1162-1 ! 72 (! 997). 5. Michaels, A.S., Chandrasekaran, S.IC, and Shaw, J.E., AIChEJ21:985-996 (1975). 6. Heisig, M., Lieckfeldt, R., Witrum, G., Mazurkevich, G., and Lee, G., Pharm. Res. 13:421-426 (1996). 7. Charalambopoulou, G.C., et al.,. Pharm. Res. 17:1085-1091 (2000). 8. Talreja, P.S., K!eene, N.K., Pickens, W., Wang, T.-F., and Kasting, G.B., submitted (2001). 9. Christophers, E. and Kligman, A.M., J. Invest. DermatoL 42:407-409 (1964). 10. Wang, T.-F., Talreja, P., Kasfing, G.B., and Nitsche, J.M. AIChE Annual Meeting. 2000. Los Angeles. 11. Robbins, C.R. and Fernee, K.M., J. Soc. Cosmet. Chem. 34:21-34 (1983).
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)