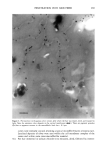
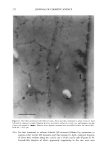
SILICONE VEHICLES FOR ALOE VERA 257 Table II The Influence of the Concentration of Emulsifier Formulation 3 Formulation 4 Formulation 5 Formulation 6 DC 245 n-decane H20 Abil EM 9O Abil WE O9 Abil EM 90 Abil WE O9 24.6% 24.6% 23.3% 23.3% 0.37% 0.37% 0.3% 0.3% 73.98% 73.98% 70.98% 70.98% 1% -- -- 1% 5% -- -- 5% chloromethane (12). The pH of the formulas selected was determined and found to be constant over time at a value of pH 5.5 + 0.5. MICROPHOTOGRAPHIC STUDY It is well known that the smaller the droplet size, the greater the stability of the system. Therefore, to study stability as a function of time and temperature, the formulations were characterized by determining the droplet size via light microscopy (Olympus BX 40 microscope coupled with an Olympus SC 35 camera). Figures la and lb are photographs obtained from preparations with Abil EM 90 sur- factant at 1% and 5% and Figures 2a and 2b are photographs of the Abil WE 09 surfactant at 1% and 5%. A 5% concentration was found to result in small droplets, regardless of the surfactant, although the photos of Abil EM 90 reveal greater homo- geneity and even smaller droplet size. Moreover, the study of the influence of time and temperature has revealed Abil EM 90 to provide slightly more stable preparations. Figure 3 shows the distribution of the droplet size for both Abil EM 90 and Abil WE 09, clearly revealing the above-mentioned characteristic of a smaller droplet size at greater concentrations of surfactant. In Abil EM 9, 80% of droplets range in size from 1-9 pm, compared to 75% for Abil WE 09. When the surfactant concentration is lower, the increase in droplet size is considerable, as can be seen. Abil EM 90 1% Abil EM 90 5% (b) Figure 1. Microphotographs of the droplets of the formulations with Abil EM 90 at 1% (a) and 5% (b)
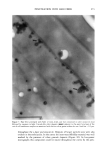
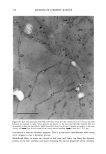
258 JOURNAL OF COSMETIC SCIENCE Abil WE 09 1% (b) Abil WE 09 5% Figure 2. Microphotographs of the formulations with Abil WE 09 at 1% (a) and 5% (b). Photographs of the study on the influence of temperature show slight changes in the 5 % surfactant preparations after one month at 40øC, consisting of a scant increase in droplet size, which was somewhat greater in the Abil WE 09 formula (Figure 4) than in the Abil EM 90 formula (Figure 5). At 60øC, however, the destruction of the system was greater and affected both preparations equally (Figure 6). RHEOLOGICAL STUDY Rheological studies are of great importance in evaluating physicochemical properties, as lOO 8o 40 1 - 9 gm 10 - 30 tam Figure 3. Graph comparing droplet size of the preparations with l•, Abil EM 90 5% El, Abil EM 90 1% •], Abil WE 09 5% and 1 Abil WE 09 1%.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)