2001 ANNUAL SCIENTIFIC SEMINAR 351 To test the adhesion properties of the NanoSal TM to human cells, we used an ex-vivo method. The HeLa cell line, that we have been chosen to determine the bioadhesive properties of the NanoSal m' is an epithelial-like cell line, originally derived from a carcinoma of the cervix. To test the adherence of NanoSal TM to the cell surface, HeLa cells were plated at a density of 2x l0 s cells per dish (35 mm) in 2 ml medium. On the following day, the NanoSal TM were dispersed in 1 ml medium. The medium in which the cells were cultured were aspirated and replaced immediately with the NanoSal TM containing medium. The NanoSal TM were left to adhere to the cells by gravity for 5 min. At each time point the medium was aspirated, and the cells surface was gently rinsed twice with 2 ml medium. Evaluation of the performance of NanoSal TM from a hair shampoo was carried out by suspending the NanoSal TM slurry in a commercial shampoo base to a concentration of 5 ml/L. 5 gr of the shampoo comprising the NanoSal TM was applied to 5-gr hair swatches and rinsed well for 3 min with tap water to simulate typical application. The hair was allowed to line dry at room temperature for 48 hr prior to subjecting them to the Scanning Electron Microscope (SEM) for visual analysis. Results and Discussion The rational design of a controlled release system for skin care was directed to address several basic requirements from the aesthetic, practical and functional points of view. These issues include means to provide: good skin fell, conventional manner of application, performance that consumer can perceive and relate to, and cost effective product. We identified several major steps in the developmental process, including: 1. Mass transfer analysis to enable us to screen and select materials that retain the active during storage, and release it when it's in contact with skin. We wanted the system to be soft enough to allow the user to activate the release by pressure formed while massaging the cream onto the skin. 2. Materials that are safe, preferably selected from natural sources, and can meet the physical requirements for the process to manufacture NanoSal TM 3. Materials that retain the natural biological potency of the actives over time, stabilized active ingredients in aqueous cosmetic dermatological, and pharmaceutical compositions, over an extended period of time 4. System that can be incorporated in a typical commercial product base to result with a stable homogeneous product over time, as determined by accelerated standard stability tests 5. System adheres to skin or hair, from an application, to allow the release of the active over time following the application 6. Enhanced liposolubility of hydrophilic actives, thus facilitate uptake into cells and increase bio-availability and intracellular accumulation 7. Controlled, continuous release of effective levels of the active over an extended period of time following application 8. The release rate of the active ingredient can be synchronized with that of a "sensory marker" to convey to the consumer the product performance This paper reviews only some of the results obtained through this research, designed to develop a general prototype system. The research utilized specific actives to demonstrate the principles of the design. These principles can be applied for the design of various systems, to release of a wide range of botanical extracts, such as: peptide, vitamins etc. However, the properties of the active will determine the type of materials and procedures used for the preparation of NanoSal TM. We characterized the mass transfer coefficient of a model active through several blends of polymers that were screened as potential matrices for our NanoSal TM system. As an experimental model for a cosmeceutical active ingredient we used a blend of botanical extracts. This blend can also be used as a "sensory marker", due to the distinct odor of the materials. The sensory marker is used to simulate and convey the release of the active to the consumer. For a specific study we utilized a blend of 3 botanicals that have distinct odor, such as: lavender, orange extract and bergamot. The odorant release rate can be measured by analytical methods, such as: GC, HPLC, weight loss, as well as by sensory methods, by evaluating odor intensity. In this experiments, we evaluated
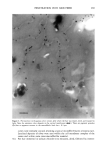
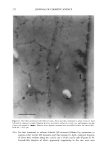
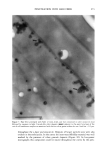
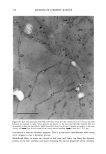
352 JOURNAL OF COSMETIC SCIENCE the release of odorant ingredients by measuring their diffusion rate to the air. In other cases, the solubility of the system in the skin is more important than the diffusion of the odorant to air. Therefore the analytical method is based on the specific need. Preparation of NanoSal TM NanoSal TM consists of a solid core, composed of a blend of hydrophobic crystalline polymers, and a high molecular weight polymeric surfactant with a charge group that is embedded on the surface of the sphere and assist in adhering the NanoSal TM to biological surfaces such as: skin, and hair:. The polymers used for the matrix are biodegradable, and made out of materials that are listed as GRAS for cosmetic application. The polymeric surfactants provide the solid core comprising the active ingredients, the bio-adhesive properties. The NanoSal TM particles diameter is between 0.01 microns and 5 microns. Particle size distribution is controlled by type of polymers, and production conditions. The NanoSal TM size and hydrophobic properties closely related to those of liposomes, and thus are used to enhance lipophilicity of actives and penetration into the deeper layers of the stratum cornium. The major benefit of the NanoSal TM over typical liposomes is stability and ability to retain materials in its semi crystalline structure that is more resistant to shear, environmental changes and temperature. Other unique properties reside from the polymeric surfactants surrounding the surfaces of the nanospheres and include: 1, enhanced retention of the active in the core, through adding another layer that retains actives from leaking out the solid core during storage. 2, the lingering polymeric chain is used as a mean to enhance adhesion onto different surfaces, and 3, prevent the spheres from coagulation. The ability of the NanoSal TM to adhere onto human cells was studied utilizing HeLa cultured cells. The adhesion of the NanoSal TM onto the cultured cells is demonstrated in Figure 1. The images indicate that the NanoSal TM adhere specifically to the HeLa cells and less to the protein coated plastic surface. Figure 1: Specific adhesion of NanoSal TM to HeLa cells. Performance of NanoSal TM in practical application The analysis and visualization of ]NTanoSal TM adhesion and disintegration into a skin tissue is under process. We focused on demonstrating the adhesion of the system onto hair from typical hair care products (i.e., shampoo and conditioners). NanoSal TM slurry was easily incorporated into a typical commercial shampoo and conditioner products, resulting with a compatible and stable blend. Hair was used as a model, because the hair surface has some properties closely related to skin, and SEM can be utilized to determine the adhesion onto the hair. Hair swatches
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)