266 JOURNAL OF COSMETIC SCIENCE a boundary to be crossed and ignores the important contribution of this layer and its component structures to the desired end result, e.g., a change in hair color. There are currently two hypotheses for the penetration of materials into the hair fiber. Each of these appears to consider the passage of molecules across the cuticle but does not answer important questions regarding fiber structure or the efficacy of formulations. The first hypothesis considers the endocuticle of each cuticle cell to be the main pathway to the cortex (1-3). However, each cuticle cell is bounded by a cell membrane complex both between cells and between the cuticle and cortex. This first approach offers no solid explanation as to how molecules exit the endocuticle and enter the cortex. This would require molecules to pass across the multilamellar cmc, and there is no obvious structural pathway. Mention is made of diffusion via the non-keratinous regions of the fiber, but the cmc is viewed as being physically too small to play a major role in penetration. The second approach considers the cell membrane complex between cuticle cells as the primary route to the cortex (4,5). This is argued on the fact that the cmc of the cuticle, particularly the cmc cement, and cortex are a continuous pathway throughout the fiber. However, this approach does not adequately explain the rapid loss of molecules such as hair dyes from the fiber during routine washing, i.e., how they get out of the cortex and back along the cmc. In these studies I have used novel derivations of established electron microscopy tech- niques. These have been combined with early photographic chemistry to place electron- dense material within hair structures. This can only occur if the molecules have free access into those structures. When studying fiber penetration it is important to under- stand fully the morphology of the fiber. Only then can one begin to interpret the data and understand that the postulated mechanisms are, in fact, complementary. METHODS 1. Using routine light microscopy, single fibers containing a continuous medulla were placed dry under a cover slip. The ends of the fibers protruded from the edges of the cover slip. No mounting media was used. Water was delivered under the cover slip, with continuous observation of the fiber at room temperature. 2. Hair fibres were treated before processing for transmission electron microscopy with one or more of the following reagents: uranyl acetate, lead citrate, aqueous silver nitrate, or ammoniacal silver nitrate (6), all at room temperature. 3. Hairs were immersed in 10% aqueous silver nitrate in the dark for either two minutes or ten minutes at room temperature, rinsed, dried, and then exposed to light. 4. Hair fibers were immersed in aqueous silver nitrate for ten minutes at room tem- perature. The solution was then made alkaline with sodium hydroxide to precipitate silver hydroxide within the fiber also at room temperature. Fibers were then pro- cessed for TEM. 5. Hair fibers were immersed in aqueous sodium chloride (2-60 minutes), rinsed in DI water, and dried. The fibers were then immersed in aqueous silver nitrate (2-60 minutes) to deposit silver chloride within the fiber, rinsed, and dried. The fibers were then exposed to bright light to produce metallic silver inside the fiber and processed for TEM. All steps were performed at room temperature. 6. Processing for TEM: No fixation was used. Fibers were embedded in Spur's low- viscosity resin. For most experiments no post staining of grids was used.
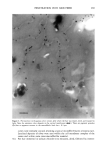
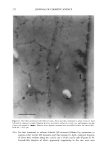
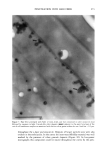
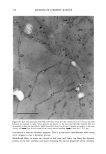
PENETRATION INTO HAIR FIBER 267 RESULTS 1. Medullated hair fibers viewed by light microscopy in the presence of water: With the dry fiber, the medulla was easily observed as a light or dark structure de- pending on the natural hair color. Observation was made easier with grey hair. Within one minute of the application of water, progressive changes in the optical appearance of the medulla could be seen. In localized areas the walls of the medulla changed in contrast relative to its previous dry state and relative to the cortex (Figure 1). 2. Using the typical combination of aqueous uranyl acetate (five minutes) and aque- ous lead citrate (three minutes) to treat whole fibers, all sections showed typical cuticle staining (Figure 2). This included intense staining of the endocuticle and the delta layer of the cmc. The exocuticle was more lightly stained. Virtually no stain was observed in the a-layer. All cortical cells showed obvious staining, particularly of the cell nuclear remnants and inter-macrofibrillar material. 3. Hairs immersed in aqueous silver nitrate, rinsed, dried, and then exposed to light: For hairs immersed for two minutes, little to no staining of the cuticle was observed. Contrast in the cortex was too low to observe a typical microfibril/matrix composite structure. Silver grains were evident on the melanin granules. For hairs immersed for ten minutes, a similar result was found, i.e., no definitive contrast in any of the hair structures. Extensive deposits of silver grains were present in the cortex cell membranes (Figure 3). All of the pigment granules showed fine deposits of silver. 4. For hair fibers immersed in aqueous silver nitrate and then made alkaline with NaOH, all hairs showed morphological staining typical of arnmoniacal silver ß . • . ., • . t30 • t60 •. . •. Figure 1. Single medullated hair immediately after addition of water (t o) and after 60 seconds (t6o). Light micrograph. Changes in the appearance of the medulla due to hydration can be seen along the individual walls of the medulla (•--,•).
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)