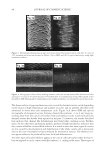
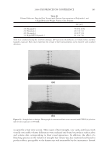
J. Cosmet. Sci., 60, 205–215 (March/April 2009) 205 A new oxidant for hair coloring JENNIFER MARSH, R. MARC DAHLGREN, COLIN CLARKE, JONATHAN STONEHOUSE, and CHRIS NUNN, Procter & Gamble, Miami Valley Innovation Center, 11810 East Miami River Road, Cincinnati, OH 45069 ( J.M., R.M.D.), and Procter & Gamble, Rusham Park Technical Centre, Whitehall Lane, Egham, Surrey, TW20 9NW, UK (C.C., J.S., C.N.). Synopsis Coloring hair using a level 3 permanent colorant involves two processes, lightening the underlying mela- nin and formation of the colored chromophores inside the hair. In typical in-market products the oxidant used to achieve these changes is hydrogen peroxide buffered at pH 10 with an alkalizer such as ammonium hydroxide. A new oxidant has been developed based on the combination of ammonium carbonate, hydrogen peroxide and glycine at pH 9 that can match the lightening and color performance of the current oxidant. It has the advantage that both the carbonate and hydrogen peroxide concentrations can be changed to alter the lighten- ing performance making it a more fl exible oxidant. This allows the capability to lighten the hair in a shorter time, or with lower hydrogen peroxide levels. This paper discusses the key oxidizing species that are present in both systems and the mechanisms of mela- nin lightening. In addition, the lightening performance will be assessed as a function of time, pH, hydrogen peroxide concentration and carbonate concentration. The importance of glycine to the oxidant is also described along with a proposal for its mechanism of action. It has been demonstrated that the addition of glycine can control the undesired formation of carbonate radi- cals that can be generated from the oxidant. The control of these radicals enables the oxidant to deliver excel- lent lightening with no negatives in fi ber damage vs. conventional oxidants. INTRODUCTION Level-three permanent hair colorant products typically consist of three major compo- nents: the oxidant which is hydrogen peroxide, the alkalizer which is typically either ammonia or ethanolamine and the dye precursors (1,2). The fi nal pH of the system is between pH 9.8 and 10.3. The role of the oxidant is three-fold. It must decolorize the melanin pigment to lighten the underlying color of the hair. It must also bleach previ- ously deposited synthetic color in the hair and it must couple together the dye precursors to form the colored chromophores. It is this balance of melanin lightening vs. deposition of synthetic color inside the hair that gives the hair its fi nal color. The lightening is very important to coloring products to enable the consumer to achieve the shade that she de- sires. This is critical for the blonde shades where very little dye is deposited and most of the fi nal color is due to the melanin lightening. However, it is also very important for
JOURNAL OF COSMETIC SCIENCE 206 achieving good gray coverage without the negative of the shade going too dark. In this case, the oxidant lightens the underlying pigment and previously deposited color thus allowing for the addition of dye to achieve good gray coverage with no decrease in the darkness of the shade and no build-up of dye and darkening over multiple usage occa- sions. The lighting is also important for the achievement of vibrant shades, for example the red shades. However, the oxidant is also responsible for the fi ber changes that can occur as the hair is colored for multiple treatments leading to consumer negatives such as poor shine, poor feel and reduced strength (3). The search for an alternative oxidant that can lighten hair but also have no negatives on fi ber damage has long been one of interest to companies that develop hair coloring products. In this paper we explore the alternative oxidant of am- monium carbonate, hydrogen peroxide and glycine buffered at pH 9.0 and we describe its proposed mechanism of action. This oxidant participates in the same color forming reactions as conventional oxidants and it can be used to match the typical range of shades. However, this color formation chemistry will not be discussed as part of this paper. EXPERIMENTAL Caucasian untreated mixed hair (medium brown), obtained from a commercial source (IHIP, New York), was formed into swatches (16 cm, 1.5 g) and used for all the testing. A range of cream formulation products were made to test the lightening effi ciency of the new oxidant as a function of pH, ammonium carbonate concentration and hydrogen per- oxide concentration. A concentrated cream base was made using 5.0% of Crodafos CES® (a mix of cetearyl alcohol, dicetylphosphate and ceteth-10 phosphate) which was dis- solved in hot DI water (85°C), neutralized with sodium hydroxide and then allowed to slowly cool. The ammonium carbonate, glycine and hydrogen peroxide were added to the remaining water and then mixed into the concentrated cream base until it was homoge- nous. The pH was adjusted with sodium hydroxide. The same concentrated cream base was used to make the formulations with the conven- tional oxidant. In this system the pH was adjusted with acetic acid. The fi nal cream formulation (4 g of product per g of hair) was thoroughly applied to the hair and then kept for 30 minutes at a controlled temperature of 30°C. The color of the hair was measured with a bench top Minolta CM3600D spectrophoto m- eter. Lab values were calculated under D65 illuminant, 10° observer, specular included. The lightening (dL) was calculated as the difference in L value between the fi nal color and the starting color on the untreated hair. 13 C NMR spectra were acquired on a Bruker Avance spectrometer operating at a 13 C fre- quency of 100.6 MHz. All spectra were acquired with an inverse gated 13 C experiment with a relaxation delay of 30 s, SW of 249 ppm and FID acquisition time of 1.3 s and 16 scans of spectral averaging were used. In the data presented later, in order to boost sensi- tivity, 10% of the quoted mass of ammonium carbonate solutions was replaced by 99% 13 C atom percent enriched sodium carbonate (Sigma Aldrich).
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)