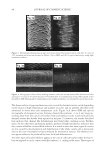
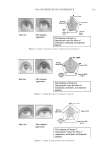
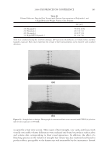
JOURNAL OF COSMETIC SCIENCE 240 hair, forming a clear fi lm. Insoluble actives (e.g. silicones) are effectively trapped in the coacervate and deposited along with the polymer. While PQ10 and cationic guar are the most widely used conditioning polymers, the large variety of monomers available to make synthetic polymers has led to an explosion in the development of synthetic cationic polymers. Previous work with PQ10 and the hydro- phobe modifi ed PQ24 and PQ67 showed signifi cant effects of changing parameters such as molecular weight, charge density and hydrophobic substitution on polymer perfor- mance (Figure 1) (4,8,9). Using these structure function relationships, polymers can be fi ne tuned to obtain customized effi cacy for deposition, wet comb and dry conditioning properties. In the case of the naturally derived polymers, the structural changes are limited by the polymer backbone provided by nature. In contrast, the backbone for synthetic polymers can be changed and is limited only by the availability of monomers, giving the synthetic chemist additional “levers” to alter polymer performance. One of the key differences between natural and synthetic polymers is backbone fl exibility in solution. Natural polymers tend to have a more rigid backbone while synthetic poly- mers are more fl exible. While polymer performance is related to the ability to form coacervate and subsequently deposit on hair, the conformation of the polymer on the substrate and the surface properties it imparts to the hair are of equal, if not greater, importance to overall conditioning properties. It is unclear how backbone fl exibility affects polymer conformation and properties on the substrate. According to the Personal Care Product Council website (http://www.personalcare council . org/), there were 88 polyquaternium polymers with INCI names in 2008 compared with 42 Figure 1. Cationic hydroxyethyl cellulose (PQ10, PQ24, and PQ67) can be modifi ed via molecular weight, charge density and hydrophobic substitution. Backbone composition can be designed for synthetic polymers.
2008 TRI/PRINCETON CONFERENCE 241 in 1999, more than doubling in ten years. This total does not include cationic polymers and blends that do not have “polyquaternium” in the INCI name. Most of these polymers are synthetic derivatives. The large number of polymers is a refl ection of the wide variety of monomers available and the numerous combinations that can be synthesized and vari- ables that can be changed within a polymer family (e.g. molecular weight, charge density, monomer ratios, hydrophobic substitution, etc) (3,10,11). Several cationic polymers, par- ticularly those that contain polyvinyl pyrrolidone, are designed for styling while others are designed for conditioning properties and still others for a combination of properties. In this study, about one hundred polymers were synthesized in an effort to develop struc- ture function relationships of synthetic cationic polymers as conditioning agents. Variables such as monomer content and ratios, molecular weight and charge density were evalu- ated. In addition, an experimental cationic polymer designed for a different industrial application was found to have signifi cantly improved deposition characteristics compared with current commercial polymers. EXPERIMENTAL MATERIALS All materials were used as received: Cationic polymers. PQ10 was from Amerchol Corporation (Piscataway, NJ). Experimental synthetic polymers were synthesized by Dow Wolff Cellulosics and The Dow Chemical Company to specifi cations. Commercial synthetic polymers were obtained from Nalco (Tarrytown, NY), BASF (Florham Park, NJ), Rhodia (Cranbury, NJ), and CIBA (Tarry- town, NY). Surfactants. Sodium laureth sulfate, SLES-2, cocoamidopropyl betaine, CAPB, disodium cocamphodiacetate, and DSCADA were all from Cognis (Cincinnati, OH). Silicone. Silicone emulsion (particle size ~200 nm) was from Dow Corning (Midland, MI) Hair. Hair was from International Hair Importers and Products (White Plains, NY). METHODS Wet combing. Wet combing was measured using a Dia-Stron Miniature Tensile Tester. The force required to pull a comb through a wet hair tress treated with surfactant alone and then surfactant plus polymer was measured. Five grams of hair was washed with 0.5 grams of a shampoo, and rinsed at constant temperature. The difference in force required to comb the hair tress before and after treatment was reported as the wet comb reduction. Coacervate study. Haze measurements were performed using a hazemeter two minutes after diluting the test formulation 2.5 to 20 times with water and mixing at room temperature. Silicone deposition. The amount of silicone deposited on hair after shampooing was mea- sured. Five grams of hair was washed with 1 gram of a shampoo, and rinsed at constant temperature. Hair was extracted with a 1:1 mixture of methyl butyl ketone and toluene. Atomic absorption spectroscopy was used to measure silicone content reporting μg silicone/g hair.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)