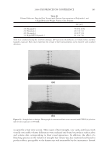
JOURNAL OF COSMETIC SCIENCE 250 REFERENCES (1) W. Li and S. L. P. Jordan, Cationic cellulosic polymers with multifunctional and outstanding perfor- mance for personal care, Cosmet. Toiletr., 1, 1–4 (2003). (2) R. Schueller and P. Romanowski, “Introduction to Conditioning Agents for Hair and Skin”, in Condi- tioning Agents for Hair and Skin, R. Schueller and P. Romanowski, Eds. (Marcel Dekker, New York, 1999), pp. 1–12. (3) B. Idson, “Polymers as Conditioning Agents for Hair and Skin,” in Conditioning Agents for Hair and Skin, R. Schueller and P. Romanowski, Eds. (Marcel Dekker, New York, 1999), pp. 251–280. (4) S. L. Jordan, R. L. Kreeger, X. Zhang, T. V. Drovetskaya, C. B. Davis, J. L. Amos, S. E. Gabelnick, S. Zhou, W. Li, E. F. DiAntonio, and A. A. Protonentis, “Effect of Hydrophobic Substitution on Cationic Conditioning Polymers,” in Cosmetic Nanotechnology Polymers and Colloids in Cosmetics, S. E. Morgan, K. O. Havelka, and R. Y. Lochhead, Eds. (ACS Symposium Series, 2007), pp. 59–71. (5) R. Y. Lochhead, “Shampoos,” in The Chemistry and Manufacture of Cosmetics, 3rd ed., M. L. Schlossman, Ed. (Allured Publishing Corporation, Carol Stream, IL, 2002), vol. 2, pp. 277–326. (6) R. Y. Lochhead, Conditioning shampoo, Soap, Cosmet. Chem. Spec., 42–49 (October 1992). (7) E. D. Goddard, “Polymer/Surfactant Interaction in Applied Systems,” in Principles of Polymer Science and Technology in Cosmetics and Personal Care, E. D. Goddard and J. V. Grubber, Eds. (Marcel Dekker, New York, 1999), pp. 181. (8) T. V. Drovetskaya, R. L. Kreeger, J. L. Amos, C. B. Davis, and S. Zhou, Effects of low-level hydrophobic substitution on conditioning properties of cationic cellulosic polymers in shampoo systems, J. Cosmet. Sci., 55(Suppl.), S195–S205 (2004). (9) T. V. Drovetskaya, E. F. DiAntonio, R. L. Kreeger, J. L. Amos, and D. P. Frank, New high-charge den- sity hydrophobically modifi ed cationic HEC polymers for improved co-deposition of benefi t agents and serious conditioning for problem hair, J. Cosmet. Sci., 58, 421–434 (2007). (10) R. Y. Lochhead and J. V. Gruber, “Appendix: Encyclopedia of Polymers and Thickeners for Cosmetics,” in Principles of Polymer Science and Technology in Cosmetics and Personal Care, E. D. Goddard and J. V. Grub- ber, Eds. (Marcel Dekker, New York, 1999), pp. 571–655. (11) J. V. Gruber, “Synthetic Polymers in Cosmetics,” in Principles of Polymer Science and Technology in Cosmetics and Personal Care, E. D. Goddard and J. V. Grubber, Eds. (Marcel Dekker, New York, 1999), pp. 217– 274. (12) W. Li, J. Amos, S. Jordan, A. Theis, and C. Davis, Selecting the optimum silicone particle size/cationic polymer structure to maximize shampoo conditioning performance, J. Cosmet. Sci., 57(2), 178–180 (2006).
J. Cosmet. Sci., 60, 251–259 (March/April 2009) 251 The mechanics of fi xatives as explained by polymer composite principles DENISE WADE RAFFERTY, JOSEPH ZELLIA, DANIEL HASMAN, and JOHN MULLAY, Lubrizol Advanced Materials, Inc., Noveon® Consumer Specialties, 9911 Brecksville Rd., Brecksville, OH 44141-3201. Synopsis Polymer composite principles are shown to explain the mechanism and performance of fi xative-treated hair tresses. This concept is illustrated using cassia and experimental cassia hydroxypropyltrimonium chloride derivatives at two charge densities. Correlations are drawn between polymer fi lm and fi xative performance properties, and the primary mechanisms behind the performance of each polymer are suggested. The cationic charge density affects the adhesion and cohesion of these polymers, and the contributions of these two proper- ties to performance are shown. It is also shown that the relationship between cationic charge density and fi xative stiffness of these polymers is dependent on the relative humidity (RH) of the test. The lower charge density polymer yields higher tress stiffness than the higher charge density polymer at 50% RH, but this trend is reversed at 90% RH. A hy- pothesis is offered in explanation of this phenomenon, relating adhesion and cohesion to the performance of fi xative-hair composites. At high humidity, moisture can plasticize the polymers, reducing the cohesive strength, so electrostatic attraction and thus adhesion becomes the dominant force. Evidence to support this hypothesis is given. INTRODUCTION Hair fi xative gels are widely used to create and maintain a variety of hairstyles. Two im- portant properties desired in hair gel products are stiffness and hold, which are controlled by the fi xative polymer in the formulation. To satisfy increasing consumer demands, per- formance with respect to these properties must be improved relative to the current fi xa- tive polymers. Understanding the science behind fi xative gel-treated hair is essential to achieving these improvements. A hair fi xative gel is a cosmetic product however, its performance is governed by polymer composite mechanisms. When a gel is applied to the hair, a polymer-fi ber composite is created that is morphologically similar to high performance fi ber composites (1) used in load-bearing applications. The differences between fi xative-treated hair and industrial fi ber composites are primarily in the mode of fabrication and the performance specifi cations. Correspondence should be addressed to Denise Wade Rafferty.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)