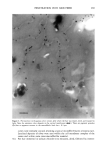
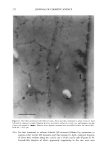
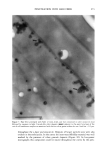
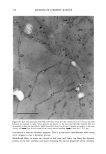
OCCLUSIVE PROPERTIES OF SLN 323 an important role for the occlusion of a system. Depending upon the needs of a carrier system, SLN dispersions and formulations with high or low occlusive characteristics can be produced and designed in a controlled way. Our studies show that the conditions for optimized occlusivity are a particle size of about 200 nm, a minimum lipid mass of 3.7 mg/cm 2, and a high degree of crystallinity of the lipid matrix. REFERENCES (9) (10) (11) (1) R. H. Mfiller and J. S. Lucks, Arzneistoffrilger aus resten Lipidteilchen, Feste Lipidnanosphilren (SLN), European Patent No. 0605497 (1996). (2) R. H. Mfiller, W. Mehnert, J. S. Lucks, C. Schwarz, A. zur Mfihlen, H. Weyhers, C. Freitas, and D. Rfihl, Solid lipid nanoparticles (SLN)--An alternative colloidal carrier system for controlled drug delivery, Eur. J. Pharm. Biopharm., 41, 62-69 (1995). (3) R. H. Mfiller, K. Milder, and S. Gohla, Solid lipid nanoparticles (SLN) for controlled drug delivery--A review of the state of the art, Eur. J. Pharm. Bzopharm., 50, 161-177 (2000). (4) R.H. Mfiller and A. Dingier, The next generation after the liposomes: Solid lipid nanoparticles (SLN TM, Lipopearls•M) as dermal carrier in cosmetics, Eurocosmetics, 7/8, 19-26 (1998). (5) V. Jenning, Feste Lipid-Nanopartikel (SLN) al• Tr•ersystem fiir die dermale App/ikation yon Retinol: Wirkstoffinkorporation, -freisetzung und Strukmr, Ph.D. Thesis, FU Berlin (1999). (6) A. Dingler, G. Hildebrand, H. Niehus, and R. H. Mfiller, Cosmetic anti-aging formulation based on vitamin E-loaded solid lipid nanoparticles, Proe. Int. Symp. Control. Release Bioact. Mater., 25,433-434 (1998). (7) V. Jenning, S. Gohla, and R. H. Mfiller, Solid lipid nanoparticles (SLN): Effect of homogenization parameters on drug stability, Proc. 2 " World Meeting APGI/APV, 619-620 (1998). (8) V. Jenning, A. Gysler, M. Schilfer-Korting, and S. Gohla, Vitamin A loaded solid lipid nanoparticles for topical use: Occlusive properties and drug penetration into porcine skin, Eur. J. Pharm. Biopharm., 49, 211-218 (2000). V. Jenning, M. Schilfer-Korting, and S. Gohla, Vitamin A loaded solid lipid nanoparticles for topical application: Drug release properties. J. Control. Release, 66, 115-126 (2000). B. W. Barry, "Skin Transport," in Dermatologieal Formulations, B. W. Barry, Ed. (Marcel Dekker, New York, 1983), pp. 95-117. J. Ziegenmeyer, "Biopharmazeutische Aspekte bei der Anwendung von Dermatika," in Dermatika, R. Niedner and J. Ziegenmeyer, Eds. (Wissenschaftliche Verlagsgesellschaft mbH, Stuttgart, 1992), pp. 243-3O8. (12) D. E. Wurster and S. F. Kramer, Investigations of some factors influencing percutaneous absorption, J. Pharm. Sci., 50, 288-293 (1961). (13) B.W. Barry, D. Southwell, and R. Woodford, Optimization of bioavailability of topical steroids: Penetration enhancers under occlusion, J. Inve•t. Dermatol., 82, 49-52 (1984). (14) R.H. Mfiller and A. Dingier, Feste Lipid-Nanopartikel (Lipopearls TM) als neuartiger Carrier ffir kosmetische und dermatologische Wirkstoffe, Pharmazeutische Zeitung/Dermopharmazie, 49, 11-15 (1998). (15) A. Dingier, Feste Lipid-Nanopartikel als kolloidale Wirkstoffir•ersysteme zur dermalen Applikation, Ph.D. Thesis, FU Berlin (1998). (16) S. A. Wissing, K. Milder, and R. H. Mfiller, Solid lipid nanoparticles (SLN)--A novel carrier for UV blockers, Eur. J. Pharm. Biopharm. (submitted). (17) R. H. Mfiller, A. Dingier, T. Schneppe, and S. Gohla, "Large Scale Production of Solid Lipid Nano- particles (SLN) and Nanosuspensions (DissoCubes)," in D. Wise, Ed., Handbook of Pharmaceutical Release Technology (Marcel Dekker, New York, 2000), pp. 359-376. (18) T. de Vringer, Topical preparation containing a suspension of solid lipid particles, European Patent No. 91200664 (1992). (19) S. A. Wissing and R. H. Mfiller, A novel sunscreen system based on tocopherol acetate incorporated into solid lipid nanoparticles (SLN), Int. J. Cosm. Sci. (in press).
324 JOURNAL OF COSMETIC SCIENCE (20) H. Bunjes, K. Westesen, and M. H. J. Koch, Crystallization tendency and polymorphic transitions in triglyceride nanoparticles, I,t. J. Pharm., 129, 159-173 (1996). (21) De•tsches Arz,eib•ch 1998 (Deutscher Apotheker Verlag, Stuttgart, & Govi Verlag, Frankfurt, 1998). (22) M. A. Thevenin, J. L. Grosslord, and M. C. Poelman, Sucrose esters/cosurfactant microemulsion sys- tems for transdermal delivery: Assessment of bicontinuous structures, Ira. J, Pharm., 137, 177-186 (1996). (23) G. Marti-Mestres and F. Nielloud, "Main Surfactants Used in the Pharmaceutical Field," in Pharma- ceutical Emulsions and S•spensions, F. Nielloud and G. Marti-Mestres, Eds. (Marcel Dekker, New York, Basel, 2000), pp. 1-18. (24) B. Brancq, Development and trends of sugar derived surfactants, Seifen, (Sle, Fette, Wachse, 118, 905-906 (1992).
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)